Citrate Buffer Table Form


What is the citrate buffer table?
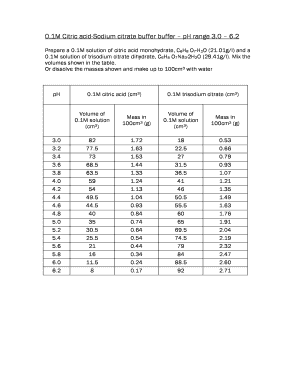
The citrate buffer table is a valuable resource that provides information on the composition and pH range of sodium citrate buffer solutions. It typically includes various concentrations of sodium citrate and citric acid, which are essential for preparing buffers used in biochemical and molecular biology applications. Understanding the citrate buffer table allows researchers to select the appropriate buffer composition for their specific experiments, ensuring optimal conditions for enzyme activity and other biochemical processes.
How to use the citrate buffer table
Using the citrate buffer table involves identifying the desired pH for your experiment and selecting the corresponding sodium citrate and citric acid concentrations. The table outlines the pH values achieved with different ratios of these components, enabling users to prepare a buffer that meets their experimental needs. By following the concentrations listed in the table, researchers can create a buffer solution that maintains the required pH, which is crucial for the stability of various biological molecules.
Steps to complete the citrate buffer table
Completing the citrate buffer table requires careful measurement and mixing of sodium citrate and citric acid. Here are the steps to follow:
- Determine the target pH for your buffer solution.
- Consult the citrate buffer table to find the corresponding concentrations of sodium citrate and citric acid.
- Weigh the appropriate amounts of sodium citrate and citric acid using a precise scale.
- Dissolve the solids in a specified volume of distilled water, ensuring complete mixing.
- Adjust the pH if necessary, using small amounts of citric acid or sodium hydroxide until the desired pH is achieved.
- Store the prepared buffer solution in a clean, labeled container for future use.
Key elements of the citrate buffer table
The citrate buffer table includes several key elements that are essential for effective buffer preparation. These elements typically consist of:
- Concentration: The specific amounts of sodium citrate and citric acid needed for each pH level.
- pH Range: The range of pH values that can be achieved with the given concentrations.
- Volume: The total volume of buffer solution that can be prepared from the specified concentrations.
- Temperature: The temperature at which the pH values are measured, as pH can vary with temperature.
Examples of using the citrate buffer table
Examples of using the citrate buffer table can provide practical insights into its application. For instance, if a researcher needs a buffer at pH 6.0, they would refer to the table to find the appropriate concentrations of sodium citrate and citric acid. If the table indicates that a mixture of 0.1 M sodium citrate and 0.1 M citric acid achieves this pH, the researcher would prepare the buffer accordingly. Such examples illustrate how the citrate buffer table serves as a guide for preparing buffers tailored to specific experimental requirements.
Legal use of the citrate buffer table
Legal use of the citrate buffer table pertains to ensuring compliance with regulations surrounding laboratory practices and safety standards. Researchers should be aware of any institutional guidelines regarding the preparation and use of chemical buffers. Additionally, proper documentation of buffer preparation, including the use of the citrate buffer table, may be necessary for regulatory compliance and quality assurance in research settings.
Quick guide on how to complete citrate buffer table
Effortlessly Prepare Citrate Buffer Table on Any Device
Digital document management has become increasingly favored by businesses and individuals alike. It serves as an ideal environmentally friendly substitute for conventional printed and signed documents, allowing you to access the necessary form and securely save it online. airSlate SignNow equips you with all the tools required to efficiently create, modify, and eSign your documents without delays. Manage Citrate Buffer Table on any device using airSlate SignNow's Android or iOS applications, and enhance any document-related task today.
The Easiest Method to Modify and eSign Citrate Buffer Table with Ease
- Obtain Citrate Buffer Table and click Get Form to begin.
- Utilize the features we offer to complete your form.
- Emphasize essential sections of the documents or conceal sensitive information with tools specifically designed for that purpose by airSlate SignNow.
- Generate your eSignature using the Sign tool, which takes mere seconds and holds the same legal validity as a conventional wet ink signature.
- Verify all details and click on the Done button to finalize your edits.
- Choose your preferred method to share your form, whether by email, SMS, invite link, or download it directly to your computer.
Say goodbye to lost or misplaced files, cumbersome form searching, or errors that require printing new document copies. airSlate SignNow meets your document management needs in just a few clicks from any device you prefer. Modify and eSign Citrate Buffer Table and guarantee outstanding communication at every stage of the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the citrate buffer table
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is sodium citrate buffer preparation and why is it important?
Sodium citrate buffer preparation involves mixing sodium citrate with a specific pH of distilled water to create a stabilizing solution. This buffer is crucial in biochemical experiments because it helps maintain the pH levels during reactions and optimizes enzyme activity.
-
How do I prepare a sodium citrate buffer solution?
To prepare a sodium citrate buffer solution, you typically dissolve sodium citrate in distilled water and adjust the pH using citric acid or sodium hydroxide as needed. It's essential to follow the proper concentrations depending on your specific experimental requirements for effective sodium citrate buffer preparation.
-
What are the common applications of sodium citrate buffer?
Sodium citrate buffer is widely used in biological research, particularly in enzyme assays and DNA extraction protocols. Its ability to maintain a stable pH makes sodium citrate buffer preparation a preferred choice for experiments requiring precise conditions.
-
Are there any alternatives to sodium citrate buffer?
Yes, there are several alternatives to sodium citrate buffer, such as phosphate buffers and Tris buffers. However, sodium citrate buffer preparation is often favored for its simplicity and effectiveness in certain biochemical applications.
-
What is the cost of purchasing pre-made sodium citrate buffer solutions?
The cost of pre-made sodium citrate buffer solutions can vary based on the supplier and volume purchased, typically ranging from $50 to $200 per liter. For frequent users, preparing your own sodium citrate buffer may be more cost-effective over time.
-
Can I use sodium citrate buffer for tissue culture applications?
Yes, sodium citrate buffer can be used in tissue culture applications to maintain pH stability during various procedures. Proper sodium citrate buffer preparation helps ensure optimal conditions for cell growth and maintenance.
-
What resources are available for understanding sodium citrate buffer preparation?
Many online resources, including research articles and laboratory manuals, provide detailed guidance on sodium citrate buffer preparation. Websites dedicated to laboratory techniques also offer practical tips and protocols tailored to different applications.
Get more for Citrate Buffer Table
Find out other Citrate Buffer Table
- How To Electronic signature Utah Government Document
- How To Electronic signature Washington Government PDF
- How Can I Electronic signature New Mexico Finance & Tax Accounting Word
- How Do I Electronic signature New York Education Form
- How To Electronic signature North Carolina Education Form
- How Can I Electronic signature Arizona Healthcare / Medical Form
- How Can I Electronic signature Arizona Healthcare / Medical Presentation
- How To Electronic signature Oklahoma Finance & Tax Accounting PDF
- How Can I Electronic signature Oregon Finance & Tax Accounting PDF
- How To Electronic signature Indiana Healthcare / Medical PDF
- How Do I Electronic signature Maryland Healthcare / Medical Presentation
- How To Electronic signature Tennessee Healthcare / Medical Word
- Can I Electronic signature Hawaii Insurance PDF
- Help Me With Electronic signature Colorado High Tech Form
- How To Electronic signature Indiana Insurance Document
- Can I Electronic signature Virginia Education Word
- How To Electronic signature Louisiana Insurance Document
- Can I Electronic signature Florida High Tech Document
- Can I Electronic signature Minnesota Insurance PDF
- How Do I Electronic signature Minnesota Insurance Document