Get the Medical Device Recall Reporting Form Final pdfFiller


Understanding the Medical Device Recall Reporting Form
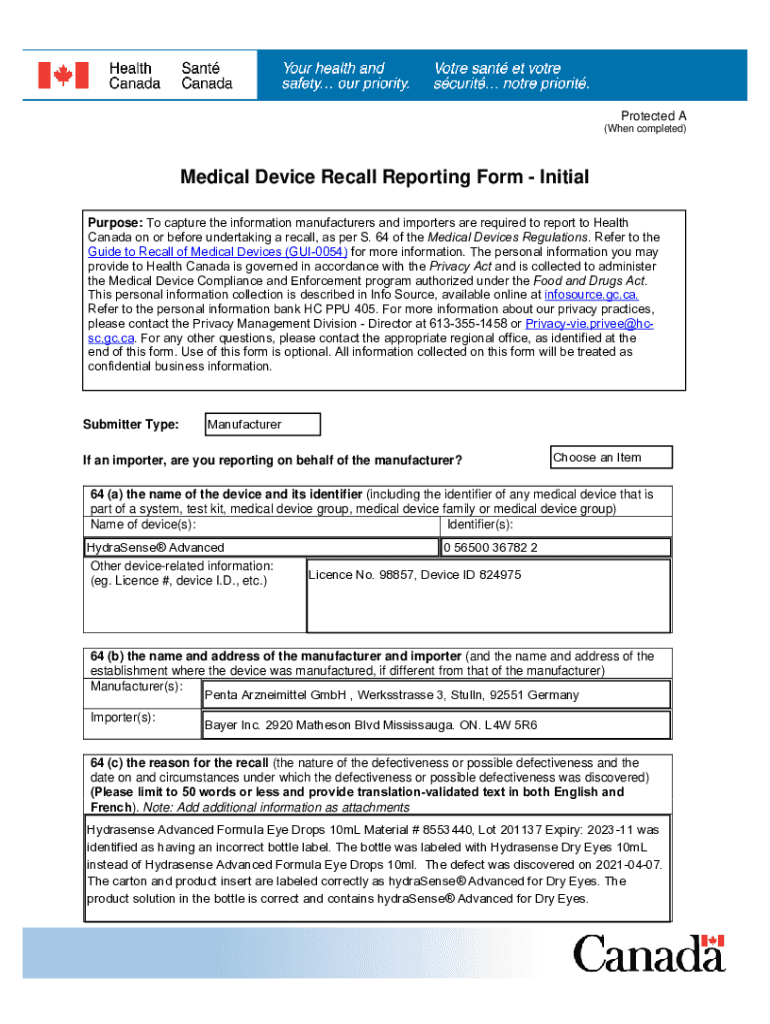
The medical device recall reporting form is a crucial document used by manufacturers, importers, and distributors to report issues related to medical devices. This form ensures that any potential risks associated with a device are communicated effectively to the appropriate regulatory bodies. The primary purpose of this form is to facilitate the safe use of medical devices and to protect public health by ensuring that any defective or unsafe devices are promptly addressed.
Key Elements of the Medical Device Recall Reporting Form
When filling out the medical device recall reporting form, it is essential to include specific details to ensure a comprehensive report. Key elements typically required include:
- Device Identification: The name, model number, and serial number of the device in question.
- Manufacturer Information: Details about the manufacturer, including name, address, and contact information.
- Reason for Recall: A clear description of the issue prompting the recall, such as safety concerns or defects.
- Recall Strategy: Information on how the recall will be conducted, including communication plans and corrective actions.
Steps to Complete the Medical Device Recall Reporting Form
Completing the medical device recall reporting form involves several steps to ensure accuracy and compliance. Follow these steps:
- Gather all necessary information about the medical device, including its identification and manufacturer details.
- Clearly outline the reason for the recall, providing specific details about the safety concerns.
- Detail the recall strategy, including how the recall will be communicated to affected parties.
- Review the form for completeness and accuracy before submission.
Legal Use of the Medical Device Recall Reporting Form
The medical device recall reporting form must be used in compliance with federal regulations set forth by the Food and Drug Administration (FDA). Manufacturers are legally obligated to report any recalls promptly to ensure public safety. Failure to comply with these regulations can result in penalties, including fines and legal action. Understanding the legal implications of this form is vital for manufacturers and distributors to maintain compliance and protect public health.
Form Submission Methods
The medical device recall reporting form can be submitted through various methods, ensuring flexibility for users. Common submission methods include:
- Online Submission: Many regulatory bodies provide an online portal for submitting recall reports.
- Mail Submission: Users can also print the form and send it via postal mail to the appropriate regulatory agency.
- In-Person Submission: Some may choose to deliver the form directly to regulatory offices for immediate processing.
Examples of Using the Medical Device Recall Reporting Form
Understanding practical scenarios can help clarify the use of the medical device recall reporting form. Examples include:
- A manufacturer discovers a defect in a surgical instrument that could pose a risk to patients and submits a recall report.
- An importer identifies that a batch of medical devices does not meet safety standards and initiates a recall.
- A distributor learns of adverse events associated with a device and promptly files a report to alert regulatory authorities.
Quick guide on how to complete get the medical device recall reporting form final pdffiller
Effortlessly Prepare Get The Medical Device Recall Reporting Form Final PdfFiller on Any Device
Digital document management has become increasingly favored by companies and individuals alike. It serves as an ideal environmentally-friendly alternative to traditional printed and signed documents, allowing you to find the correct form and securely store it online. airSlate SignNow equips you with all the tools necessary to create, modify, and electronically sign your documents swiftly without delays. Manage Get The Medical Device Recall Reporting Form Final PdfFiller on any device with airSlate SignNow's Android or iOS applications and simplify any document-related task today.
The Easiest Way to Alter and Electronically Sign Get The Medical Device Recall Reporting Form Final PdfFiller without Hassle
- Find Get The Medical Device Recall Reporting Form Final PdfFiller and click Acquire Form to begin.
- Utilize the tools we offer to fill out your form.
- Emphasize important sections of your documents or obscure sensitive information with the tools that airSlate SignNow specifically provides for that purpose.
- Create your signature using the Signature tool, which takes just seconds and carries the same legal validity as a conventional wet signature.
- Review the information and click the Complete button to retain your changes.
- Choose how you would like to send your form, via email, text message (SMS), invitation link, or download it to your computer.
Eliminate concerns about lost or misplaced documents, tedious form searches, or errors that necessitate printing new copies. airSlate SignNow addresses all your document management needs in just a few clicks from your preferred device. Modify and electronically sign Get The Medical Device Recall Reporting Form Final PdfFiller to ensure outstanding communication at every stage of your form preparation process using airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the get the medical device recall reporting form final pdffiller
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
Get more for Get The Medical Device Recall Reporting Form Final PdfFiller
- Application for reactivation of a business permit for an electrical njconsumeraffairs form
- 137486 form
- Repatriation waiver form
- Purchase and sales agreement and deposit receipt new form
- Ally ira contribution form
- Cif ncs hydration parent permission slips form
- Model release agreement template form
- Month to month commercial lease agreement template form
Find out other Get The Medical Device Recall Reporting Form Final PdfFiller
- How Do I eSignature Alaska Life Sciences Presentation
- Help Me With eSignature Iowa Life Sciences Presentation
- How Can I eSignature Michigan Life Sciences Word
- Can I eSignature New Jersey Life Sciences Presentation
- How Can I eSignature Louisiana Non-Profit PDF
- Can I eSignature Alaska Orthodontists PDF
- How Do I eSignature New York Non-Profit Form
- How To eSignature Iowa Orthodontists Presentation
- Can I eSignature South Dakota Lawers Document
- Can I eSignature Oklahoma Orthodontists Document
- Can I eSignature Oklahoma Orthodontists Word
- How Can I eSignature Wisconsin Orthodontists Word
- How Do I eSignature Arizona Real Estate PDF
- How To eSignature Arkansas Real Estate Document
- How Do I eSignature Oregon Plumbing PPT
- How Do I eSignature Connecticut Real Estate Presentation
- Can I eSignature Arizona Sports PPT
- How Can I eSignature Wisconsin Plumbing Document
- Can I eSignature Massachusetts Real Estate PDF
- How Can I eSignature New Jersey Police Document