0910 0502; Expiration Date 8312022; See PRA Statement on Page 10 Form


Understanding the FDA Form 3537
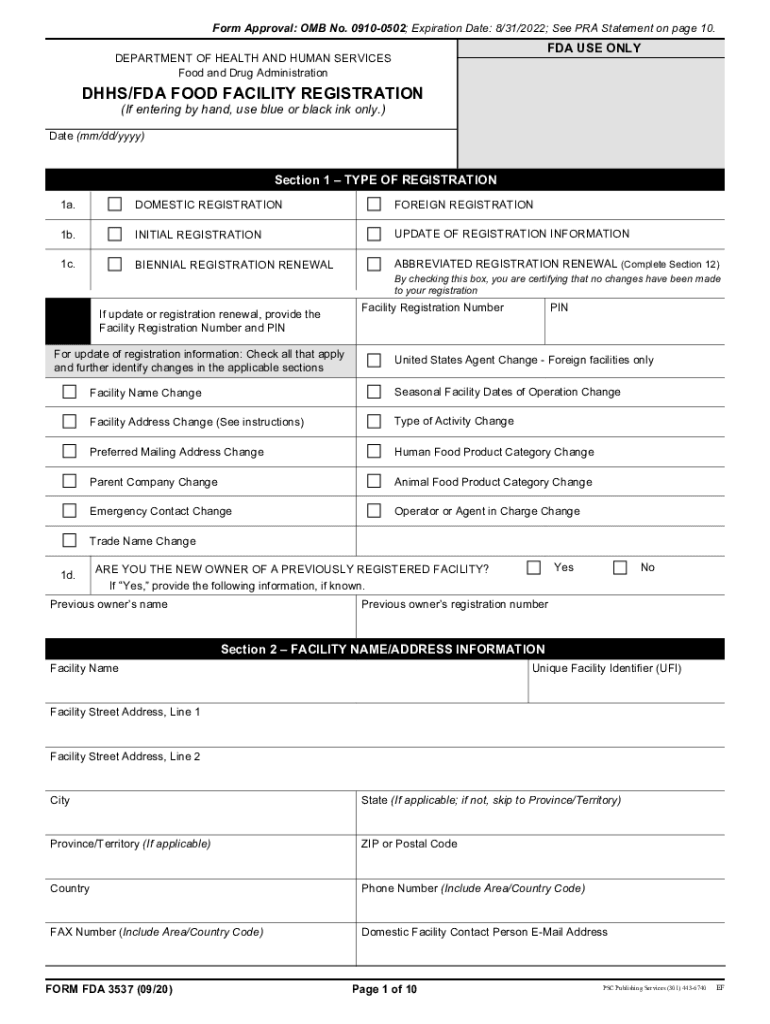
The FDA Form 3537 is essential for food facility registration in the United States. This form, also known as the Food Facility Registration form, is required for facilities that manufacture, process, pack, or hold food for consumption in the U.S. The form collects vital information about the facility, including its name, address, and the types of food products handled. It is crucial for compliance with the Food Safety Modernization Act (FSMA) and ensures that food facilities are registered with the FDA.
Steps to Complete the FDA Form 3537
Completing the FDA Form 3537 involves several key steps:
- Gather necessary information about your facility, including its legal name, physical address, and contact details.
- Identify the types of food products your facility processes or handles.
- Complete the form accurately, ensuring all sections are filled out to avoid delays.
- Submit the form electronically through the FDA's online registration system or by mail if required.
Following these steps will help ensure a smooth registration process.
Legal Use of the FDA Form 3537
The FDA Form 3537 is legally binding when completed and submitted according to the FDA's guidelines. It is essential to provide accurate information, as any discrepancies can lead to penalties or issues with compliance. The form must be signed by an authorized representative of the facility, confirming that all information is true and correct. Compliance with the registration requirements helps maintain food safety standards and protects public health.
Required Documents for Submission
When submitting the FDA Form 3537, certain documents may be required to support your registration. These may include:
- Proof of ownership or operation of the facility.
- Documentation of food safety practices implemented at the facility.
- Any relevant licenses or permits required by state or local authorities.
Having these documents ready can streamline the registration process.
Form Submission Methods
The FDA Form 3537 can be submitted through various methods. The preferred method is online submission via the FDA's Food Facility Registration Module. This method ensures faster processing and confirmation of your registration. Alternatively, you can print the form and submit it by mail. Ensure that all required information is included to avoid delays in processing.
Penalties for Non-Compliance
Failure to register your food facility using the FDA Form 3537 can result in significant penalties. Non-compliance may lead to fines, legal action, or even closure of the facility. It is essential to stay informed about registration deadlines and maintain compliance with all FDA regulations to avoid these consequences.
Quick guide on how to complete 0910 0502 expiration date 8312022 see pra statement on page 10
Complete 0910 0502; Expiration Date 8312022; See PRA Statement On Page 10 effortlessly on any device
Digital document management has gained popularity among businesses and individuals. It offers an ideal eco-friendly substitute to traditional printed and signed documents, as you can obtain the correct form and securely keep it online. airSlate SignNow equips you with all the necessary tools to create, modify, and electronically sign your documents swiftly without delays. Manage 0910 0502; Expiration Date 8312022; See PRA Statement On Page 10 on any platform with airSlate SignNow Android or iOS applications and enhance any document-centric process today.
The most efficient way to modify and electronically sign 0910 0502; Expiration Date 8312022; See PRA Statement On Page 10 without difficulty
- Locate 0910 0502; Expiration Date 8312022; See PRA Statement On Page 10 and then click Get Form to begin.
- Utilize the tools we provide to fill out your form.
- Highlight relevant sections of the documents or redact sensitive information with tools that airSlate SignNow offers specifically for that purpose.
- Create your signature with the Sign tool, which takes seconds and holds the same legal validity as a traditional wet ink signature.
- Review the information and then click on the Done button to save your modifications.
- Select how you wish to send your form, via email, text message (SMS), or invitation link, or download it to your computer.
Eliminate concerns about lost or mislaid documents, tedious form searches, or mistakes that necessitate printing new document copies. airSlate SignNow addresses your needs in document management in just a few clicks from any device of your preference. Modify and eSign 0910 0502; Expiration Date 8312022; See PRA Statement On Page 10 and ensure outstanding communication at every stage of the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the 0910 0502 expiration date 8312022 see pra statement on page 10
The way to make an eSignature for your PDF document in the online mode
The way to make an eSignature for your PDF document in Chrome
The way to make an electronic signature for putting it on PDFs in Gmail
The way to make an electronic signature straight from your mobile device
The way to make an electronic signature for a PDF document on iOS devices
The way to make an electronic signature for a PDF document on Android devices
People also ask
-
What is the process for completing a form food facility registration using airSlate SignNow?
The process for filling out a form food facility registration with airSlate SignNow is streamlined and user-friendly. You can easily upload your document, fill in the required fields, and eSign it securely. This ensures that your registration is completed quickly and accurately, meeting all necessary compliance requirements.
-
Are there any costs associated with using airSlate SignNow for form food facility registration?
Yes, airSlate SignNow offers various pricing plans to accommodate different business needs when it comes to form food facility registration. You can choose from monthly or annual plans, each providing access to features that enhance your document management and eSigning experience. Explore our pricing page for specific details.
-
What features does airSlate SignNow offer for form food facility registration?
airSlate SignNow provides features like customizable templates for form food facility registration, real-time tracking, and automated reminders. The platform also allows multiple users to collaborate on documents easily and helps ensure that all signatures are collected efficiently. These features are designed to save time and streamline your registration process.
-
How does airSlate SignNow ensure the security of my form food facility registration?
Security is a top priority at airSlate SignNow. We employ advanced encryption protocols and robust security measures to protect your form food facility registration and personal data. Additionally, our platform is compliant with global standards, ensuring that your registrations are handled safely and securely.
-
Can I integrate airSlate SignNow with other software for form food facility registration?
Absolutely! airSlate SignNow integrates seamlessly with various software and platforms, making it easier to manage your form food facility registration alongside other business functions. Popular integrations include CRM tools and document management systems, allowing you to enhance your workflow and improve efficiency.
-
What are the benefits of using airSlate SignNow for form food facility registration?
Using airSlate SignNow for your form food facility registration offers numerous benefits, including increased efficiency, reduced errors, and faster turnaround times. The eSigning capability allows you to finalize documents from anywhere, streamlining the registration process. This ultimately contributes to better compliance and operational success for your business.
-
Is airSlate SignNow suitable for small businesses handling form food facility registration?
Yes, airSlate SignNow is designed to cater to businesses of all sizes, including small businesses that need to handle form food facility registration. Our platform is cost-effective and user-friendly, making it easy for smaller teams to manage their documents without the need for extensive technical expertise. Start with a free trial to experience its benefits today.
Get more for 0910 0502; Expiration Date 8312022; See PRA Statement On Page 10
Find out other 0910 0502; Expiration Date 8312022; See PRA Statement On Page 10
- eSign Wyoming Charity Living Will Simple
- eSign Florida Construction Memorandum Of Understanding Easy
- eSign Arkansas Doctors LLC Operating Agreement Free
- eSign Hawaii Construction Lease Agreement Mobile
- Help Me With eSign Hawaii Construction LLC Operating Agreement
- eSign Hawaii Construction Work Order Myself
- eSign Delaware Doctors Quitclaim Deed Free
- eSign Colorado Doctors Operating Agreement Computer
- Help Me With eSign Florida Doctors Lease Termination Letter
- eSign Florida Doctors Lease Termination Letter Myself
- eSign Hawaii Doctors Claim Later
- eSign Idaho Construction Arbitration Agreement Easy
- eSign Iowa Construction Quitclaim Deed Now
- How Do I eSign Iowa Construction Quitclaim Deed
- eSign Louisiana Doctors Letter Of Intent Fast
- eSign Maine Doctors Promissory Note Template Easy
- eSign Kentucky Construction Claim Online
- How Can I eSign Maine Construction Quitclaim Deed
- eSign Colorado Education Promissory Note Template Easy
- eSign North Dakota Doctors Affidavit Of Heirship Now