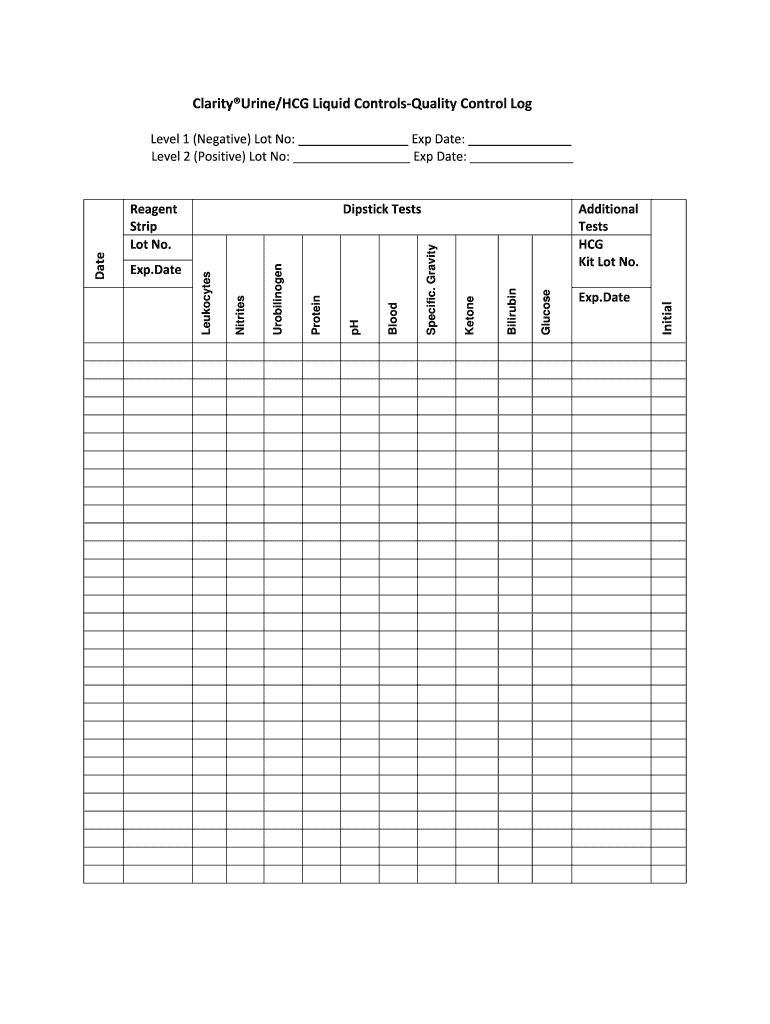
ClarityUrineHCG Liquid Controls Quality Control Log Form


What is the ClarityUrineHCG Liquid Controls Quality Control Log
The ClarityUrineHCG Liquid Controls Quality Control Log is a vital document used in laboratories to ensure the accuracy and reliability of urine pregnancy test results. This log serves as a record of the quality control tests performed on the ClarityUrineHCG liquid controls. It is essential for maintaining compliance with regulatory standards and for ensuring that test results are valid and trustworthy. The log typically includes details such as the date of testing, control lot numbers, results of the quality control tests, and any corrective actions taken if results fall outside acceptable ranges.
How to use the ClarityUrineHCG Liquid Controls Quality Control Log
Using the ClarityUrineHCG Liquid Controls Quality Control Log involves several straightforward steps. First, ensure that you have the appropriate quality control materials and that they are within their expiration dates. Next, record the date and lot number of the controls being tested in the log. Perform the quality control tests according to the manufacturer’s instructions, documenting the results in the log. If any results do not meet the expected ranges, note the corrective actions taken to resolve the issue. Regularly reviewing this log helps maintain laboratory standards and ensures ongoing compliance with quality assurance protocols.
Steps to complete the ClarityUrineHCG Liquid Controls Quality Control Log
Completing the ClarityUrineHCG Liquid Controls Quality Control Log involves a systematic approach:
- Gather necessary materials, including the quality control liquids and the log template.
- Document the date and control lot numbers at the top of the log.
- Perform the quality control tests as per the guidelines provided by the manufacturer.
- Record the results of each test accurately in the designated sections of the log.
- If results are outside the acceptable range, detail the corrective actions taken.
- Review the log for completeness and accuracy before submitting it for audit or review.
Legal use of the ClarityUrineHCG Liquid Controls Quality Control Log
The legal use of the ClarityUrineHCG Liquid Controls Quality Control Log is crucial for laboratories to demonstrate compliance with federal and state regulations. This log acts as a legal document that can be referenced in audits or inspections. To ensure its legal standing, it is important to maintain accurate records, follow proper testing protocols, and retain the logs for the required duration as stipulated by regulatory bodies. Adhering to these practices helps protect laboratories from potential legal issues and enhances the credibility of their testing processes.
Key elements of the ClarityUrineHCG Liquid Controls Quality Control Log
Key elements of the ClarityUrineHCG Liquid Controls Quality Control Log include:
- Date of testing: Essential for tracking the timeline of quality control activities.
- Control lot numbers: Important for traceability and ensuring the correct controls are used.
- Test results: Critical for evaluating the performance of the testing process.
- Corrective actions: Documenting any deviations ensures accountability and continuous improvement.
- Signature of the technician: Provides verification that the log has been completed by a qualified individual.
Examples of using the ClarityUrineHCG Liquid Controls Quality Control Log
Examples of using the ClarityUrineHCG Liquid Controls Quality Control Log can vary based on laboratory practices. For instance, a laboratory may conduct daily quality control tests to verify the accuracy of their urine pregnancy tests. Each day, the technician would fill out the log with the date, lot numbers, and results of the tests. In cases where a control result is outside the acceptable range, the technician would document the corrective actions taken, such as recalibrating the testing equipment or using a new lot of controls. This consistent documentation supports the laboratory’s commitment to quality assurance and regulatory compliance.
Quick guide on how to complete clarityurinehcg liquid controls quality control log
Prepare ClarityUrineHCG Liquid Controls Quality Control Log effortlessly on any device
Online document management has become increasingly popular among businesses and individuals. It offers an ideal eco-friendly alternative to traditional printed and signed documents, allowing you to obtain the correct form and securely store it online. airSlate SignNow provides all the resources necessary to create, modify, and electronically sign your documents swiftly without delays. Manage ClarityUrineHCG Liquid Controls Quality Control Log on any device using airSlate SignNow apps for Android or iOS and simplify any document-related tasks today.
The simplest way to alter and eSign ClarityUrineHCG Liquid Controls Quality Control Log effortlessly
- Locate ClarityUrineHCG Liquid Controls Quality Control Log and click on Get Form to begin.
- Utilize the tools we offer to complete your document.
- Select important sections of the documents or obscure sensitive information using tools that airSlate SignNow specifically offers for that purpose.
- Create your electronic signature with the Sign tool, which takes seconds and holds the same legal validity as a conventional wet ink signature.
- Review all the information carefully and click on the Done button to save your changes.
- Choose how you wish to distribute your form, whether by email, text message (SMS), invitation link, or download it to your computer.
Eliminate the hassle of lost or misplaced files, tedious form searching, or errors that necessitate printing new document copies. airSlate SignNow meets your document management needs in just a few clicks from any device you prefer. Alter and eSign ClarityUrineHCG Liquid Controls Quality Control Log to ensure excellent communication at all stages of your form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the clarityurinehcg liquid controls quality control log
The best way to make an electronic signature for a PDF in the online mode
The best way to make an electronic signature for a PDF in Chrome
The best way to create an eSignature for putting it on PDFs in Gmail
The best way to create an electronic signature from your smart phone
How to generate an eSignature for a PDF on iOS devices
The best way to create an electronic signature for a PDF file on Android OS
People also ask
-
What is a quality control log?
A quality control log is a systematic record used to document the results of quality control processes. It helps businesses monitor compliance with standards and identify areas of improvement. Using a quality control log enhances product reliability and ensures consistent quality.
-
How can airSlate SignNow help with maintaining a quality control log?
airSlate SignNow provides an efficient way to create, send, and eSign quality control logs. Its easy-to-use interface ensures that all team members can access and fill out logs seamlessly. This streamlines the quality control process and ensures that all records are up-to-date and compliant.
-
Are there any costs associated with using airSlate SignNow for quality control logs?
Yes, airSlate SignNow offers various pricing plans to suit different business needs, including features for managing quality control logs. The pricing is designed to be cost-effective while providing robust solutions for document management and eSigning. You can find a plan that fits your budget and quality control needs.
-
What features does airSlate SignNow offer for creating a quality control log?
airSlate SignNow includes features like customizable templates, automated workflows, and real-time collaboration tools for quality control logs. You can easily edit and personalize logs to match your specific requirements. This makes it simple to capture essential quality control data and improve overall tracking.
-
Can I integrate airSlate SignNow with other software tools for managing quality control logs?
Absolutely! airSlate SignNow offers integrations with numerous business applications, making it easy to incorporate quality control logs into your existing workflows. This ensures that data from various platforms can be centralized, enhancing the overall efficiency of your quality control processes.
-
What are the benefits of using a digital quality control log?
Using a digital quality control log streamlines the documentation process, reduces paper usage, and enhances accessibility. With airSlate SignNow, you can easily store and retrieve logs, leading to better data management and compliance. Digital logs also facilitate quicker team collaboration and timely updates.
-
Is airSlate SignNow user-friendly for teams unfamiliar with digital quality control logs?
Yes, airSlate SignNow is designed with user experience in mind, making it intuitive for teams of all tech levels. The platform provides easy navigation and helpful resources to guide users in managing their quality control logs. This ensures that everyone on your team can effectively use the system without extensive training.
Get more for ClarityUrineHCG Liquid Controls Quality Control Log
Find out other ClarityUrineHCG Liquid Controls Quality Control Log
- How To Electronic signature Arkansas Construction Word
- How Do I Electronic signature Arkansas Construction Document
- Can I Electronic signature Delaware Construction PDF
- How Can I Electronic signature Ohio Business Operations Document
- How Do I Electronic signature Iowa Construction Document
- How Can I Electronic signature South Carolina Charity PDF
- How Can I Electronic signature Oklahoma Doctors Document
- How Can I Electronic signature Alabama Finance & Tax Accounting Document
- How To Electronic signature Delaware Government Document
- Help Me With Electronic signature Indiana Education PDF
- How To Electronic signature Connecticut Government Document
- How To Electronic signature Georgia Government PDF
- Can I Electronic signature Iowa Education Form
- How To Electronic signature Idaho Government Presentation
- Help Me With Electronic signature Hawaii Finance & Tax Accounting Document
- How Can I Electronic signature Indiana Government PDF
- How Can I Electronic signature Illinois Finance & Tax Accounting PPT
- How To Electronic signature Maine Government Document
- How To Electronic signature Louisiana Education Presentation
- How Can I Electronic signature Massachusetts Government PDF