Fda Form 481


What is the FDA Form 481?
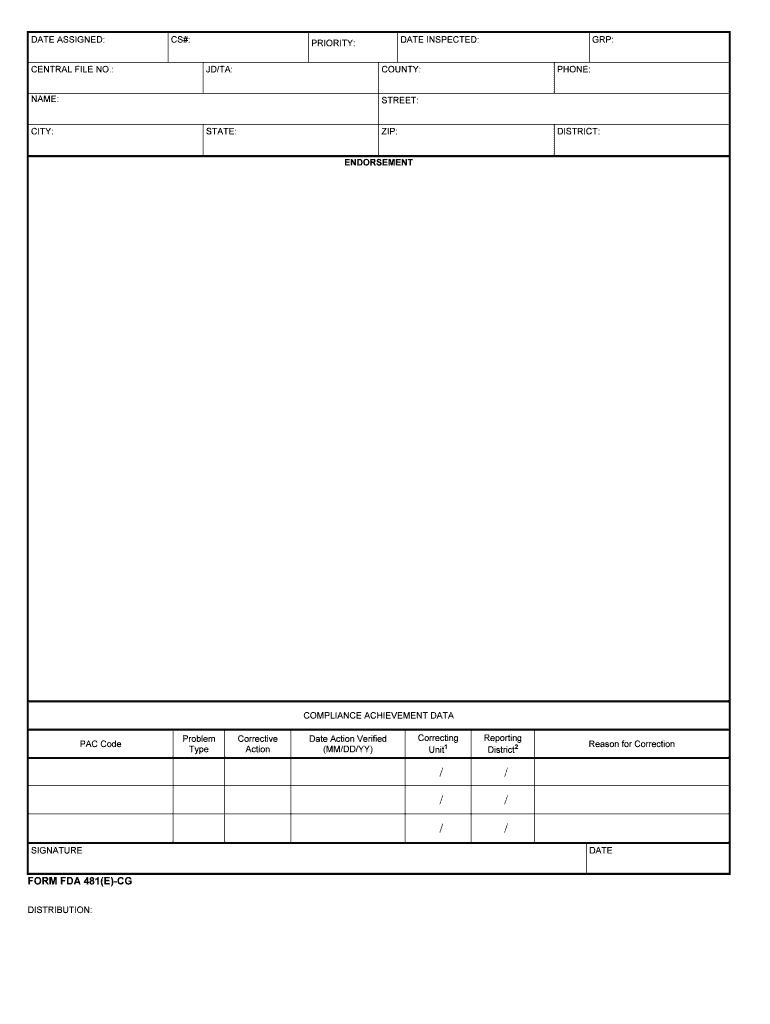
The FDA Form 481 is a crucial document used by the U.S. Food and Drug Administration (FDA) to collect information related to the management of investigational new drugs (INDs). This form is essential for ensuring compliance with federal regulations governing the clinical investigation of new pharmaceuticals. It serves as a formal request for the initiation of clinical trials and includes details about the study protocol, the investigational product, and the qualifications of the investigators involved. Understanding the purpose of this form is vital for researchers and sponsors engaged in drug development.
Steps to Complete the FDA Form 481
Completing the FDA Form 481 requires careful attention to detail to ensure compliance and accuracy. Here are the key steps:
- Gather necessary information: Collect all relevant data regarding the investigational drug, including its chemical composition, proposed indications, and any previous research findings.
- Fill out the form: Input the required information in the designated fields, ensuring that all sections are completed accurately. Pay special attention to the study protocol and investigator qualifications.
- Review the form: Double-check all entries for accuracy and completeness. It is advisable to have a colleague review the form as well.
- Submit the form: Follow the appropriate submission methods, whether online or by mail, as specified by the FDA guidelines.
How to Obtain the FDA Form 481
The FDA Form 481 can be obtained directly from the FDA's official website. It is available as a downloadable PDF document, allowing users to print and fill it out manually or complete it electronically. Ensure that you are using the most recent version of the form to avoid any issues with compliance. Additionally, the FDA provides guidance documents that can help clarify the requirements for completing the form.
Key Elements of the FDA Form 481
Understanding the key elements of the FDA Form 481 is essential for successful completion. The form typically includes:
- Study title: A concise title that reflects the nature of the investigation.
- Investigational product information: Details about the drug being studied, including its formulation and dosage.
- Investigator information: Names and qualifications of the principal investigator and any co-investigators.
- Study protocol summary: A brief overview of the study design, objectives, and methodology.
- Compliance statement: A declaration of adherence to FDA regulations and ethical standards.
Form Submission Methods
The FDA Form 481 can be submitted through various methods, depending on the specific requirements outlined by the FDA. The primary submission options include:
- Online submission: Many forms can be submitted electronically via the FDA's electronic submission system, which allows for faster processing and tracking.
- Mail submission: If online submission is not feasible, the form can be printed and mailed to the appropriate FDA office. Ensure that you follow the mailing instructions precisely to avoid delays.
- In-person submission: In some cases, researchers may choose to deliver the form directly to an FDA office. This method allows for immediate confirmation of receipt.
Legal Use of the FDA Form 481
The legal use of the FDA Form 481 is governed by federal regulations that mandate its use for the initiation of clinical trials. It is critical for researchers to ensure that all information provided is truthful and accurate, as any discrepancies may lead to legal repercussions or delays in the approval process. Compliance with the FDA's guidelines not only facilitates the approval of clinical trials but also protects the rights and safety of study participants.
Quick guide on how to complete usfda forms 481
Discover the easiest method to complete and endorse your Fda Form 481
Are you still spending time preparing your official documents on paper instead of handling them online? airSlate SignNow offers a superior way to finalize and endorse your Fda Form 481 and analogous forms for public services. Our intelligent electronic signature tool provides everything necessary to work on documents swiftly and in compliance with official standards - robust PDF editing, management, protection, signing, and sharing tools are all readily accessible within a user-friendly interface.
Only a few steps are needed to fill out and endorse your Fda Form 481:
- Upload the editable template to the editor using the Get Form button.
- Verify the information you need to enter in your Fda Form 481.
- Move between the fields with the Next button to ensure nothing is overlooked.
- Utilize Text, Check, and Cross tools to fill in the fields with your details.
- Update the content with Text boxes or Images from the upper toolbar.
- Emphasize what is essential or Conceal fields that are no longer relevant.
- Press Sign to generate a legally binding electronic signature using any method you prefer.
- Include the Date next to your signature and complete your task with the Done button.
Store your completed Fda Form 481 in the Documents folder of your profile, download it, or send it to your preferred cloud storage. Our tool also offers flexible form sharing options. There’s no need to print your templates when you need to submit them to the appropriate public office - do it via email, fax, or by requesting a USPS “snail mail” delivery from your account. Give it a try now!
Create this form in 5 minutes or less
FAQs
-
Do military members have to pay any fee for leave or fiancee forms?
NOOOOOOO. You are talking to a military romance scammer. I received an email from the US Army that directly answers your question that is pasted below please keep reading.I believe you are the victim of a military Romance Scam whereas the person you are talking to is a foreign national posing as an American Soldier claiming to be stationed overseas on a peacekeeping mission. That's the key to the scam they always claim to be on a peacekeeping mission.Part of their scam is saying that they have no access to their money that their mission is highly dangerous.If your boyfriend girlfriend/future husband/wife is asking you to do the following or has exhibited this behavior, it is a most likely a scam:Moves to private messaging site immediately after meeting you on Facebook or SnapChat or Instagram or some dating or social media site. Often times they delete the site you met them on right after they asked you to move to a more private messaging siteProfesses love to you very quickly & seems to quote poems and song lyrics along with using their own sort of broken language, as they profess their love and devotion quickly. They also showed concern for your health and love for your family.Promises marriage as soon as he/she gets to state for leave that they asked you to pay for.They Requests money (wire transfers) and Amazon, iTune ,Verizon, etc gift cards, for medicine, religious practices, and leaves to come home, internet access, complete job assignments, help sick friend, get him out of trouble, or anything that sounds fishy.The military does provide all the soldier needs including food medical Care and transportation for leave. Trust me, I lived it, you are probably being scammed. I am just trying to show you examples that you are most likely being connned.Below is an email response I received after I sent an inquiry to the US government when I discovered I was scammed. I received this wonderful response back with lots of useful links on how to find and report your scammer. And how to learn more about Romance Scams.Right now you can also copy the picture he gave you and do a google image search and you will hopefully see the pictures of the real person he is impersonating. this doesn't always work and take some digging. if you find the real person you can direct message them and alert them that their image is being used for scamming.Good Luck to you and I'm sorry this may be happening to you. please continue reading the government response I received below it's very informative. You have contacted an email that is monitored by the U.S. Army Criminal Investigation Command. Unfortunately, this is a common concern. We assure you there is never any reason to send money to anyone claiming to be a Soldier online. If you have only spoken with this person online, it is likely they are not a U.S. Soldier at all. If this is a suspected imposter social media profile, we urge you to report it to that platform as soon as possible. Please continue reading for more resources and answers to other frequently asked questions: How to report an imposter Facebook profile: Caution-https://www.facebook.com/help/16... < Caution-https://www.facebook.com/help/16... > Answers to frequently asked questions: - Soldiers and their loved ones are not charged money so that the Soldier can go on leave. - Soldiers are not charged money for secure communications or leave. - Soldiers do not need permission to get married. - Soldiers emails are in this format: john.doe.mil@mail.mil < Caution-mailto: john.doe.mil@mail.mil > anything ending in .us or .com is not an official email account. - Soldiers have medical insurance, which pays for their medical costs when treated at civilian health care facilities worldwide – family and friends do not need to pay their medical expenses. - Military aircraft are not used to transport Privately Owned Vehicles. - Army financial offices are not used to help Soldiers buy or sell items of any kind. - Soldiers deployed to Combat Zones do not need to solicit money from the public to feed or house themselves or their troops. - Deployed Soldiers do not find large unclaimed sums of money and need your help to get that money out of the country. Anyone who tells you one of the above-listed conditions/circumstances is true is likely posing as a Soldier and trying to steal money from you. We would urge you to immediately cease all contact with this individual. For more information on avoiding online scams and to report this crime, please see the following sites and articles: This article may help clarify some of the tricks social media scammers try to use to take advantage of people: Caution-https://www.army.mil/article/61432/< Caution-https://www.army.mil/article/61432/> CID advises vigilance against 'romance scams,' scammers impersonating Soldiers Caution-https://www.army.mil/article/180749 < Caution-https://www.army.mil/article/180749 > FBI Internet Crime Complaint Center: Caution-http://www.ic3.gov/default.aspx< Caution-http://www.ic3.gov/default.aspx> U.S. Army investigators warn public against romance scams: Caution-https://www.army.mil/article/130...< Caution-https://www.army.mil/article/130...> DOD warns troops, families to be cybercrime smart -Caution-http://www.army.mil/article/1450...< Caution-http://www.army.mil/article/1450...> Use caution with social networking Caution-https://www.army.mil/article/146...< Caution-https://www.army.mil/article/146...> Please see our frequently asked questions section under scams and legal issues. Caution-http://www.army.mil/faq/ < Caution-http://www.army.mil/faq/ > or visit Caution-http://www.cid.army.mil/ < Caution-http://www.cid.army.mil/ >. The challenge with most scams is determining if an individual is a legitimate member of the US Army. Based on the Privacy Act of 1974, we cannot provide this information. If concerned about a scam you may contact the Better Business Bureau (if it involves a solicitation for money), or local law enforcement. If you're involved in a Facebook or dating site scam, you are free to contact us direct; (571) 305-4056. If you have a social security number, you can find information about Soldiers online at Caution-https://www.dmdc.osd.mil/appj/sc... < Caution-https://www.dmdc.osd.mil/appj/sc... > . While this is a free search, it does not help you locate a retiree, but it can tell you if the Soldier is active duty or not. If more information is needed such as current duty station or location, you can contact the Commander Soldier's Records Data Center (SRDC) by phone or mail and they will help you locate individuals on active duty only, not retirees. There is a fee of $3.50 for businesses to use this service. The check or money order must be made out to the U.S. Treasury. It is not refundable. The address is: Commander Soldier's Records Data Center (SRDC) 8899 East 56th Street Indianapolis, IN 46249-5301 Phone: 1-866-771-6357 In addition, it is not possible to remove social networking site profiles without legitimate proof of identity theft or a scam. If you suspect fraud on this site, take a screenshot of any advances for money or impersonations and report the account on the social networking platform immediately. Please submit all information you have on this incident to Caution-www.ic3.gov < Caution-http://www.ic3.gov > (FBI website, Internet Criminal Complaint Center), immediately stop contact with the scammer (you are potentially providing them more information which can be used to scam you), and learn how to protect yourself against these scams at Caution-http://www.ftc.gov < Caution-http://www.ftc.gov > (Federal Trade Commission's website)
-
Why don't schools teach children about taxes and bills and things that they will definitely need to know as adults to get by in life?
Departments of education and school districts always have to make decisions about what to include in their curriculum. There are a lot of life skills that people need that aren't taught in school. The question is should those skills be taught in schools?I teach high school, so I'll talk about that. The typical high school curriculum is supposed to give students a broad-based education that prepares them to be citizens in a democracy and to be able to think critically. For a democracy to work, we need educated, discerning citizens with the ability to make good decisions based on evidence and objective thought. In theory, people who are well informed about history, culture, science, mathematics, etc., and are capable of critical, unbiased thinking, will have the tools to participate in a democracy and make good decisions for themselves and for society at large. In addition to that, they should be learning how to be learners, how to do effective, basic research, and collaborate with other people. If that happens, figuring out how to do procedural tasks in real life should not provide much of a challenge. We can't possibly teach every necessary life skill people need, but we can help students become better at knowing how to acquire the skills they need. Should we teach them how to change a tire when they can easily consult a book or search the internet to find step by step instructions for that? Should we teach them how to balance a check book or teach them how to think mathematically and make sense of problems so that the simple task of balancing a check book (which requires simple arithmetic and the ability to enter numbers and words in columns and rows in obvious ways) is easy for them to figure out. If we teach them to be good at critical thinking and have some problem solving skills they will be able to apply those overarching skills to all sorts of every day tasks that shouldn't be difficult for someone with decent cognitive ability to figure out. It's analogous to asking why a culinary school didn't teach its students the steps and ingredients to a specific recipe. The school taught them about more general food preparation and food science skills so that they can figure out how to make a lot of specific recipes without much trouble. They're also able to create their own recipes.So, do we want citizens with very specific skill sets that they need to get through day to day life or do we want citizens with critical thinking, problem solving, and other overarching cognitive skills that will allow them to easily acquire ANY simple, procedural skill they may come to need at any point in their lives?
-
What happens to all of the paper forms you fill out for immigration and customs?
Years ago I worked at document management company. There is cool software that can automate aspects of hand-written forms. We had an airport as a customer - they scanned plenty and (as I said before) this was several years ago...On your airport customs forms, the "boxes" that you 'need' to write on - are basically invisible to the scanner - but are used because then us humans will tend to write neater and clearer which make sit easier to recognize with a computer. Any characters with less than X% accuracy based on a recognition engine are flagged and shown as an image zoomed into the particular character so a human operator can then say "that is an "A". This way, you can rapidly go through most forms and output it to say - an SQL database, complete with link to original image of the form you filled in.If you see "black boxes" at three corners of the document - it is likely set up for scanning (they help to identify and orient the page digitally). If there is a unique barcode on the document somewhere I would theorize there is an even higher likelihood of it being scanned - the document is of enough value to be printed individually which costs more, which means it is likely going to be used on the capture side. (I've noticed in the past in Bahamas and some other Caribbean islands they use these sorts of capture mechanisms, but they have far fewer people entering than the US does everyday)The real answer is: it depends. Depending on each country and its policies and procedures. Generally I would be surprised if they scanned and held onto the paper. In the US, they proably file those for a set period of time then destroy them, perhaps mining them for some data about travellers. In the end, I suspect the "paper-to-data capture" likelihood of customs forms ranges somewhere on a spectrum like this:Third world Customs Guy has paper to show he did his job, paper gets thrown out at end of shift. ------> We keep all the papers! everything is scanned as you pass by customs and unique barcodes identify which flight/gate/area the form was handed out at, so we co-ordinate with cameras in the airport and have captured your image. We also know exactly how much vodka you brought into the country. :)
-
How do you fill out tax forms?
I strongly recommend purchasing a tax program, Turbo tax, H&R block etc.These programs will ask you questions and they will fill out the forms for you.You just print it out and mail it in. (with a check, if you owe anything)I used to use an accountant but these programs found more deductions.
-
Do I have to fill out the census forms?
Yes, you do. Census helps the government/private companies know the number and the types of people in your town/state/country. When you provide information, you get better service.If you're worried about your personal information getting leaked, don't be. A census usually only requires your name and the no. of people in your house (depends on the scale of the census)You got to nothing to lose anyway. Just give the information. It'll take less than 5 minutes.
-
How do I fill out 2013 tax forms?
I hate when people ask a question, then rather than answer, someone jumps in and tells them they don't need to know--but today, I will be that guy, because this is serious.Why oh why do you think you can do this yourself?Two things to consider:People who get a masters degree in Accounting then go get a CPA then start doing taxes--only then do some of them start specializing in international accounting. I've taught Accounting at the college-level, have taken tax classes beyond that, and wouldn't touch your return.Tax professionals generally either charge by the form or by the hour. Meaning you can sit and do this for 12 hours, or you can pay a CPA by the hour to do it, or you can go to an H&R Block that has flat rates and will do everything but hit Send for free. So why spend 12 hours doing it incorrectly, destined to worry about the IRS putting you in jail, bankrupting you, or deporting you for the next decade when you can get it done professionally for $200-$300?No, just go get it done right.
-
How many application forms does a person need to fill out in his/her lifetime?
As many as you want to !
Create this form in 5 minutes!
How to create an eSignature for the usfda forms 481
How to create an electronic signature for your Usfda Forms 481 in the online mode
How to make an eSignature for the Usfda Forms 481 in Chrome
How to generate an electronic signature for putting it on the Usfda Forms 481 in Gmail
How to make an eSignature for the Usfda Forms 481 straight from your smartphone
How to create an electronic signature for the Usfda Forms 481 on iOS devices
How to make an electronic signature for the Usfda Forms 481 on Android
People also ask
-
What is FDA Form 481 and why is it important?
FDA Form 481 is a crucial document used for the submission of information related to the administration of investigational new drug applications. It ensures compliance with FDA regulations, making it essential for businesses in the pharmaceutical and life sciences industries. Utilizing airSlate SignNow can streamline the signing process for FDA Form 481, ensuring timely and secure submissions.
-
How can airSlate SignNow help with filling out FDA Form 481?
airSlate SignNow simplifies the process of filling out FDA Form 481 by providing an intuitive interface that allows users to easily input and manage information. The platform also supports electronic signatures, which means you can quickly gather necessary approvals without delays. This efficiency is crucial for maintaining compliance with FDA deadlines.
-
What features does airSlate SignNow offer for managing FDA Form 481?
AirSlate SignNow offers features like customizable templates, secure document storage, and real-time tracking, all of which are beneficial for managing FDA Form 481. These features help ensure that your submissions are accurate, complete, and compliant with regulatory guidelines. Additionally, eSigning capabilities allow for quick turnaround times.
-
Is airSlate SignNow cost-effective for submitting FDA Form 481?
Yes, airSlate SignNow is a cost-effective solution for businesses needing to submit FDA Form 481. With flexible pricing plans, you can choose a package that fits your budget while still gaining access to essential features and tools. This affordability allows organizations of all sizes to streamline their document management processes.
-
Can I integrate airSlate SignNow with other software for FDA Form 481 submissions?
Absolutely! airSlate SignNow offers seamless integrations with various software solutions commonly used in the pharmaceutical industry, enhancing your workflow for FDA Form 481 submissions. Whether you use CRM systems or project management tools, you can easily connect them with airSlate SignNow for a more efficient document handling experience.
-
What are the benefits of using airSlate SignNow for FDA Form 481?
Using airSlate SignNow for FDA Form 481 offers numerous benefits, including increased efficiency, improved compliance, and enhanced security. The platform's user-friendly design allows for quick document preparation and signature collection, ensuring that you meet FDA submission deadlines without hassle. Moreover, built-in security features protect sensitive information throughout the process.
-
Is airSlate SignNow compliant with FDA regulations for FDA Form 481?
Yes, airSlate SignNow is compliant with FDA regulations, ensuring that your submissions of FDA Form 481 meet the necessary legal requirements. The platform adheres to industry standards for electronic signatures, making it a reliable choice for businesses in regulated environments. You can submit your documents confidently, knowing they are compliant.
Get more for Fda Form 481
Find out other Fda Form 481
- eSignature New Jersey Healthcare / Medical Credit Memo Myself
- eSignature North Dakota Healthcare / Medical Medical History Simple
- Help Me With eSignature Arkansas High Tech Arbitration Agreement
- eSignature Ohio Healthcare / Medical Operating Agreement Simple
- eSignature Oregon Healthcare / Medical Limited Power Of Attorney Computer
- eSignature Pennsylvania Healthcare / Medical Warranty Deed Computer
- eSignature Texas Healthcare / Medical Bill Of Lading Simple
- eSignature Virginia Healthcare / Medical Living Will Computer
- eSignature West Virginia Healthcare / Medical Claim Free
- How To eSignature Kansas High Tech Business Plan Template
- eSignature Kansas High Tech Lease Agreement Template Online
- eSignature Alabama Insurance Forbearance Agreement Safe
- How Can I eSignature Arkansas Insurance LLC Operating Agreement
- Help Me With eSignature Michigan High Tech Emergency Contact Form
- eSignature Louisiana Insurance Rental Application Later
- eSignature Maryland Insurance Contract Safe
- eSignature Massachusetts Insurance Lease Termination Letter Free
- eSignature Nebraska High Tech Rental Application Now
- How Do I eSignature Mississippi Insurance Separation Agreement
- Help Me With eSignature Missouri Insurance Profit And Loss Statement