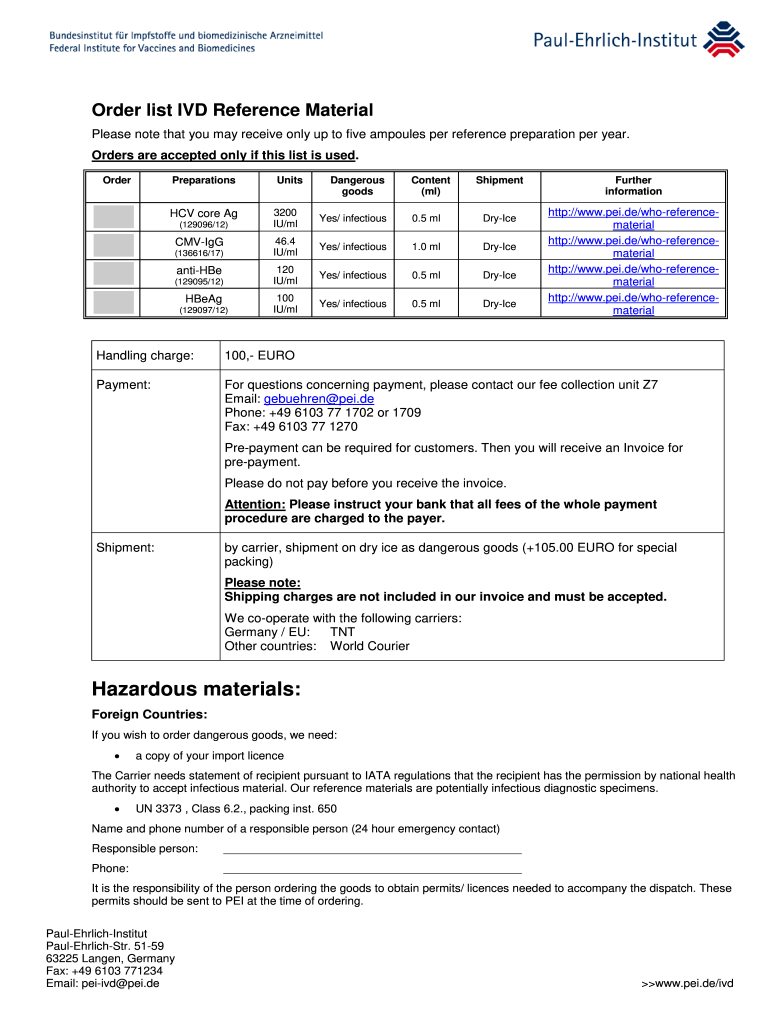
Order List IVD Reference Material Paul Ehrlich Institute Form


What is the Order List IVD Reference Material Paul Ehrlich Institute
The Order List IVD Reference Material from the Paul Ehrlich Institute serves as a crucial resource for laboratories involved in in vitro diagnostics (IVD). This list includes standardized reference materials that ensure the accuracy and reliability of diagnostic tests. These materials are essential for calibrating instruments and validating test methods, thereby enhancing the quality of healthcare services. The Paul Ehrlich Institute, as a regulatory authority, oversees the production and distribution of these reference materials, ensuring they meet rigorous scientific and regulatory standards.
How to use the Order List IVD Reference Material Paul Ehrlich Institute
Using the Order List IVD Reference Material involves several steps to ensure compliance and accuracy. First, laboratories should review the list to identify the specific reference materials required for their diagnostic tests. Once identified, they can place an order through the designated channels provided by the Paul Ehrlich Institute. It is important to ensure that the materials are used according to the guidelines provided to maintain the integrity of the testing process. Regular updates to the order list should be monitored to stay informed about new materials or changes in existing ones.
Steps to complete the Order List IVD Reference Material Paul Ehrlich Institute
Completing the order process for the Order List IVD Reference Material involves the following steps:
- Review the current Order List to identify needed reference materials.
- Gather necessary information, such as laboratory details and specific material requirements.
- Complete the order form accurately, ensuring all fields are filled in correctly.
- Submit the order through the specified method, whether online or via mail.
- Keep a copy of the submitted order for your records.
Legal use of the Order List IVD Reference Material Paul Ehrlich Institute
The legal use of the Order List IVD Reference Material is governed by regulations set forth by health authorities and the Paul Ehrlich Institute. Laboratories must ensure that they comply with all relevant laws when ordering and using these materials. This includes adhering to quality control measures and maintaining proper documentation of the materials used in diagnostic testing. Non-compliance can lead to legal repercussions and impact the validity of test results, making it essential for laboratories to stay informed about their legal obligations.
Key elements of the Order List IVD Reference Material Paul Ehrlich Institute
Key elements of the Order List IVD Reference Material include:
- Material Identification: Each reference material is clearly identified with a unique code.
- Specifications: Detailed descriptions of the materials, including their intended use and storage conditions.
- Compliance Information: Information on how the materials meet regulatory standards.
- Ordering Instructions: Clear guidelines on how to place an order and any associated costs.
Examples of using the Order List IVD Reference Material Paul Ehrlich Institute
Examples of using the Order List IVD Reference Material include:
- Calibration of laboratory equipment to ensure accurate test results.
- Validation of new diagnostic tests before they are introduced to the market.
- Quality control checks to maintain the reliability of ongoing tests.
Quick guide on how to complete order list ivd reference material paul ehrlich institute
Effortlessly Prepare Order List IVD Reference Material Paul Ehrlich Institute on Any Device
The management of online documents has become increasingly favored by businesses and individuals. It serves as an excellent environmentally friendly alternative to traditional printed and signed documents, as you can access the correct form and securely store it online. airSlate SignNow provides you with all the tools you need to create, edit, and eSign your documents swiftly without delays. Manage Order List IVD Reference Material Paul Ehrlich Institute on any platform with the airSlate SignNow Android or iOS applications and streamline any document-related process today.
How to Edit and eSign Order List IVD Reference Material Paul Ehrlich Institute with Ease
- Obtain Order List IVD Reference Material Paul Ehrlich Institute and click Get Form to begin.
- Utilize the tools we offer to complete your document.
- Highlight pertinent sections of your documents or obscure sensitive information with tools provided by airSlate SignNow specifically for that purpose.
- Generate your eSignature using the Sign feature, which takes just seconds and holds the same legal validity as a conventional wet ink signature.
- Review all information and click on the Done button to save your changes.
- Choose your preferred method to send your form, whether by email, text message (SMS), invite link, or download it to your computer.
Eliminate the hassle of lost or misplaced documents, cumbersome form searches, or mistakes that necessitate printing new document copies. airSlate SignNow meets your document management requirements with just a few clicks from any device you choose. Edit and eSign Order List IVD Reference Material Paul Ehrlich Institute and ensure excellent communication at every phase of the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the order list ivd reference material paul ehrlich institute
The way to create an electronic signature for your PDF document in the online mode
The way to create an electronic signature for your PDF document in Chrome
How to make an electronic signature for putting it on PDFs in Gmail
How to make an electronic signature right from your mobile device
The best way to create an electronic signature for a PDF document on iOS devices
How to make an electronic signature for a PDF on Android devices
People also ask
-
What is the 'Order List IVD Reference Material Paul Ehrlich Institute'?
The 'Order List IVD Reference Material Paul Ehrlich Institute' is a comprehensive catalog of quality-controlled reference materials necessary for in vitro diagnostics. It supports labs in ensuring the accuracy and reliability of their tests. This resource is essential for maintaining compliance and enhancing the quality of diagnostic results.
-
How can I place an order for IVD Reference Material from the Paul Ehrlich Institute?
To place an order for IVD Reference Material from the Paul Ehrlich Institute, visit our website and access the 'Order List IVD Reference Material Paul Ehrlich Institute'. From there, select the materials required and follow the checkout process. Our user-friendly platform ensures a seamless ordering experience.
-
What pricing options are available for the IVD Reference Materials?
Pricing for the IVD Reference Materials from the Paul Ehrlich Institute varies based on the specific products selected. The 'Order List IVD Reference Material Paul Ehrlich Institute' provides transparent pricing and any bulk order discounts available, making it easier for laboratories to budget effectively.
-
What are the benefits of using the IVD Reference Material from the Paul Ehrlich Institute?
Using the IVD Reference Material from the Paul Ehrlich Institute ensures your diagnostic tests are accurate and reliable, leading to better patient outcomes. These materials adhere to strict regulatory standards and help in maintaining compliance with diagnostic guidelines. The 'Order List IVD Reference Material Paul Ehrlich Institute' allows labs to enhance their testing capabilities signNowly.
-
Are there any integrations available for the IVD Reference Material ordering process?
Yes, the ordering system for the 'Order List IVD Reference Material Paul Ehrlich Institute' integrates with popular laboratory information management systems (LIMS). This integration streamlines your workflow and reduces manual data entry, allowing for more efficient operations within your lab.
-
How does the quality assurance process work for the IVD Reference Materials?
The IVD Reference Materials from the Paul Ehrlich Institute undergo rigorous quality assurance protocols to ensure accuracy and reliability. Each product listed in the 'Order List IVD Reference Material Paul Ehrlich Institute' is subjected to extensive testing and validation before it signNowes your lab, ensuring that you receive only the highest quality materials.
-
Can I receive assistance while placing an order for IVD Reference Material?
Absolutely! Our customer support team is available to assist you with any queries regarding the 'Order List IVD Reference Material Paul Ehrlich Institute'. You can contact us via email or phone, and we’ll ensure that your ordering process is smooth and hassle-free.
Get more for Order List IVD Reference Material Paul Ehrlich Institute
- Underground storage tank monitoring system daily inspection form
- Tournament application form updated 10413 say soccer saysoccer
- Clallam county fire district no 2 residential burn bpermitb form
- Sewage system file review north bay mattawa conservation form
- Excelsior college transcript request form
- Arizona model plan form edward j maney chapter 13 trustee
- Official transcript request form quinsigamond community college qcc
- Dros form 53666413
Find out other Order List IVD Reference Material Paul Ehrlich Institute
- Electronic signature Wisconsin Charity Lease Agreement Mobile
- Can I Electronic signature Wisconsin Charity Lease Agreement
- Electronic signature Utah Business Operations LLC Operating Agreement Later
- How To Electronic signature Michigan Construction Cease And Desist Letter
- Electronic signature Wisconsin Business Operations LLC Operating Agreement Myself
- Electronic signature Colorado Doctors Emergency Contact Form Secure
- How Do I Electronic signature Georgia Doctors Purchase Order Template
- Electronic signature Doctors PDF Louisiana Now
- How To Electronic signature Massachusetts Doctors Quitclaim Deed
- Electronic signature Minnesota Doctors Last Will And Testament Later
- How To Electronic signature Michigan Doctors LLC Operating Agreement
- How Do I Electronic signature Oregon Construction Business Plan Template
- How Do I Electronic signature Oregon Construction Living Will
- How Can I Electronic signature Oregon Construction LLC Operating Agreement
- How To Electronic signature Oregon Construction Limited Power Of Attorney
- Electronic signature Montana Doctors Last Will And Testament Safe
- Electronic signature New York Doctors Permission Slip Free
- Electronic signature South Dakota Construction Quitclaim Deed Easy
- Electronic signature Texas Construction Claim Safe
- Electronic signature Texas Construction Promissory Note Template Online