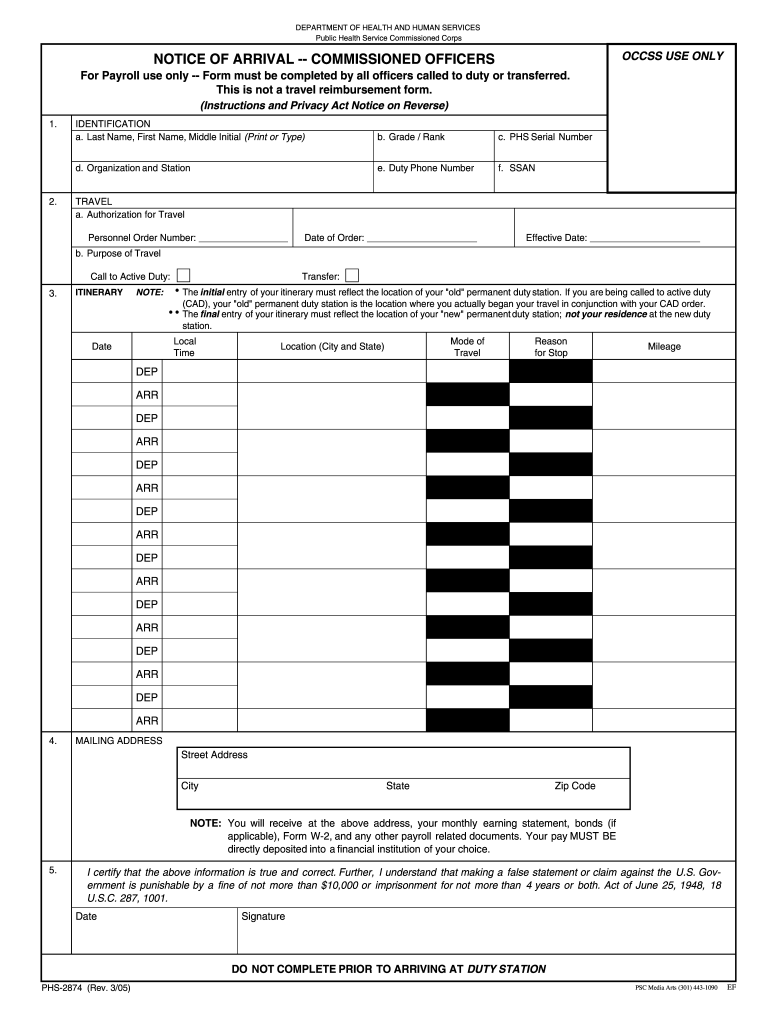
Phs 2874 2005-2026


What is the PHS 2874?
The PHS 2874, also known as the notice arrival form, is a crucial document used by commissioned officers of the Public Health Service (PHS) to formally notify their respective agencies of their arrival at a new duty station. This form serves as an official record that facilitates the transition of officers between assignments, ensuring that all necessary administrative processes are initiated upon their arrival.
The PHS 2874 is specifically designed to capture essential information about the officer, including their name, rank, and the details of their new assignment. This information is vital for maintaining accurate personnel records and ensuring that the officer receives the appropriate support and resources at their new location.
Steps to Complete the PHS 2874
Completing the PHS 2874 involves several important steps to ensure accuracy and compliance with agency requirements. Begin by gathering all necessary personal information, including your full name, rank, and the details of your new duty station. Next, fill out the required fields on the form, paying close attention to any specific instructions provided by your agency.
After completing the form, review it thoroughly to ensure that all information is correct and all required fields are filled. It may also be beneficial to consult with a supervisor or administrative officer to confirm that the form meets all agency standards. Once verified, submit the form through the designated submission method, which may include online submission, mailing, or in-person delivery.
Legal Use of the PHS 2874
The PHS 2874 is legally recognized as an official document that must be submitted by commissioned officers upon their arrival at a new duty station. Its legal validity hinges on the accurate completion and timely submission of the form. Failure to submit the PHS 2874 can lead to administrative complications, including delays in receiving benefits or support services at the new assignment.
Moreover, the information provided on the form is used for maintaining accurate personnel records within the PHS. It is essential for officers to understand the legal implications of the PHS 2874, as it not only affects their administrative status but also their eligibility for various programs and resources available to PHS officers.
How to Obtain the PHS 2874
The PHS 2874 can typically be obtained through official PHS channels, such as the agency's human resources department or online portals dedicated to officer administration. Many agencies provide downloadable versions of the form on their websites, ensuring easy access for officers preparing for a transition.
It is advisable for officers to check their agency's specific guidelines regarding the acquisition of the PHS 2874, as procedures may vary. Additionally, officers can reach out to their administrative support staff for assistance in obtaining the most current version of the form.
Form Submission Methods
Submitting the PHS 2874 can be done through various methods, depending on the agency's policies. Common submission methods include online submission via secure portals, mailing the completed form to the appropriate administrative office, or delivering it in person to ensure it is received promptly.
When submitting the form online, ensure that you are using a secure connection to protect your personal information. If mailing the form, consider using certified mail to confirm receipt. In-person submissions may provide an opportunity to clarify any questions with administrative staff, ensuring that the form is processed correctly.
Key Elements of the PHS 2874
Understanding the key elements of the PHS 2874 is essential for effective completion. The form typically includes sections for personal identification, such as name, rank, and contact information, as well as details about the new duty station, including address and reporting date.
Additionally, there may be sections requiring the officer's signature and the date of submission. Ensuring that all elements are filled out accurately is crucial, as incomplete forms can lead to delays in processing and potential administrative issues.
Quick guide on how to complete phs 2874 form
Discover the simplest method to complete and authorize your Phs 2874
Are you still spending time preparing your official documents on paper instead of doing it digitally? airSlate SignNow offers a superior approach to finalize and approve your Phs 2874 and related forms for public services. Our intelligent electronic signature solution equips you with all the tools needed to handle paperwork swiftly and in compliance with formal standards - robust PDF editing, management, security, signing, and sharing capabilities, all integrated into an intuitive interface.
Only a few steps are necessary to complete and authorize your Phs 2874:
- Upload the fillable form to the editor using the Get Form option.
- Verify the information you need to input in your Phs 2874.
- Navigate through the fields using the Next feature to ensure nothing is overlooked.
- Utilize Text, Check, and Cross tools to fill in the blanks with your information.
- Update the content with Text boxes or Images from the upper toolbar.
- Emphasize key points or Blackout irrelevant sections.
- Click on Sign to generate a legally valid electronic signature using your preferred method.
- Add the Date next to your signature and conclude your task with the Done option.
Store your completed Phs 2874 in the Documents directory of your profile, download it, or transfer it to your preferred cloud storage. Our platform also offers versatile form sharing. There's no need to print out your documents when you need to submit them to the relevant public office - send them via email, fax, or request a USPS “snail mail” delivery from your account. Try it out today!
Create this form in 5 minutes or less
FAQs
-
Do military members have to pay any fee for leave or fiancee forms?
NOOOOOOO. You are talking to a military romance scammer. I received an email from the US Army that directly answers your question that is pasted below please keep reading.I believe you are the victim of a military Romance Scam whereas the person you are talking to is a foreign national posing as an American Soldier claiming to be stationed overseas on a peacekeeping mission. That's the key to the scam they always claim to be on a peacekeeping mission.Part of their scam is saying that they have no access to their money that their mission is highly dangerous.If your boyfriend girlfriend/future husband/wife is asking you to do the following or has exhibited this behavior, it is a most likely a scam:Moves to private messaging site immediately after meeting you on Facebook or SnapChat or Instagram or some dating or social media site. Often times they delete the site you met them on right after they asked you to move to a more private messaging siteProfesses love to you very quickly & seems to quote poems and song lyrics along with using their own sort of broken language, as they profess their love and devotion quickly. They also showed concern for your health and love for your family.Promises marriage as soon as he/she gets to state for leave that they asked you to pay for.They Requests money (wire transfers) and Amazon, iTune ,Verizon, etc gift cards, for medicine, religious practices, and leaves to come home, internet access, complete job assignments, help sick friend, get him out of trouble, or anything that sounds fishy.The military does provide all the soldier needs including food medical Care and transportation for leave. Trust me, I lived it, you are probably being scammed. I am just trying to show you examples that you are most likely being connned.Below is an email response I received after I sent an inquiry to the US government when I discovered I was scammed. I received this wonderful response back with lots of useful links on how to find and report your scammer. And how to learn more about Romance Scams.Right now you can also copy the picture he gave you and do a google image search and you will hopefully see the pictures of the real person he is impersonating. this doesn't always work and take some digging. if you find the real person you can direct message them and alert them that their image is being used for scamming.Good Luck to you and I'm sorry this may be happening to you. please continue reading the government response I received below it's very informative. You have contacted an email that is monitored by the U.S. Army Criminal Investigation Command. Unfortunately, this is a common concern. We assure you there is never any reason to send money to anyone claiming to be a Soldier online. If you have only spoken with this person online, it is likely they are not a U.S. Soldier at all. If this is a suspected imposter social media profile, we urge you to report it to that platform as soon as possible. Please continue reading for more resources and answers to other frequently asked questions: How to report an imposter Facebook profile: Caution-https://www.facebook.com/help/16... < Caution-https://www.facebook.com/help/16... > Answers to frequently asked questions: - Soldiers and their loved ones are not charged money so that the Soldier can go on leave. - Soldiers are not charged money for secure communications or leave. - Soldiers do not need permission to get married. - Soldiers emails are in this format: john.doe.mil@mail.mil < Caution-mailto: john.doe.mil@mail.mil > anything ending in .us or .com is not an official email account. - Soldiers have medical insurance, which pays for their medical costs when treated at civilian health care facilities worldwide – family and friends do not need to pay their medical expenses. - Military aircraft are not used to transport Privately Owned Vehicles. - Army financial offices are not used to help Soldiers buy or sell items of any kind. - Soldiers deployed to Combat Zones do not need to solicit money from the public to feed or house themselves or their troops. - Deployed Soldiers do not find large unclaimed sums of money and need your help to get that money out of the country. Anyone who tells you one of the above-listed conditions/circumstances is true is likely posing as a Soldier and trying to steal money from you. We would urge you to immediately cease all contact with this individual. For more information on avoiding online scams and to report this crime, please see the following sites and articles: This article may help clarify some of the tricks social media scammers try to use to take advantage of people: Caution-https://www.army.mil/article/61432/< Caution-https://www.army.mil/article/61432/> CID advises vigilance against 'romance scams,' scammers impersonating Soldiers Caution-https://www.army.mil/article/180749 < Caution-https://www.army.mil/article/180749 > FBI Internet Crime Complaint Center: Caution-http://www.ic3.gov/default.aspx< Caution-http://www.ic3.gov/default.aspx> U.S. Army investigators warn public against romance scams: Caution-https://www.army.mil/article/130...< Caution-https://www.army.mil/article/130...> DOD warns troops, families to be cybercrime smart -Caution-http://www.army.mil/article/1450...< Caution-http://www.army.mil/article/1450...> Use caution with social networking Caution-https://www.army.mil/article/146...< Caution-https://www.army.mil/article/146...> Please see our frequently asked questions section under scams and legal issues. Caution-http://www.army.mil/faq/ < Caution-http://www.army.mil/faq/ > or visit Caution-http://www.cid.army.mil/ < Caution-http://www.cid.army.mil/ >. The challenge with most scams is determining if an individual is a legitimate member of the US Army. Based on the Privacy Act of 1974, we cannot provide this information. If concerned about a scam you may contact the Better Business Bureau (if it involves a solicitation for money), or local law enforcement. If you're involved in a Facebook or dating site scam, you are free to contact us direct; (571) 305-4056. If you have a social security number, you can find information about Soldiers online at Caution-https://www.dmdc.osd.mil/appj/sc... < Caution-https://www.dmdc.osd.mil/appj/sc... > . While this is a free search, it does not help you locate a retiree, but it can tell you if the Soldier is active duty or not. If more information is needed such as current duty station or location, you can contact the Commander Soldier's Records Data Center (SRDC) by phone or mail and they will help you locate individuals on active duty only, not retirees. There is a fee of $3.50 for businesses to use this service. The check or money order must be made out to the U.S. Treasury. It is not refundable. The address is: Commander Soldier's Records Data Center (SRDC) 8899 East 56th Street Indianapolis, IN 46249-5301 Phone: 1-866-771-6357 In addition, it is not possible to remove social networking site profiles without legitimate proof of identity theft or a scam. If you suspect fraud on this site, take a screenshot of any advances for money or impersonations and report the account on the social networking platform immediately. Please submit all information you have on this incident to Caution-www.ic3.gov < Caution-http://www.ic3.gov > (FBI website, Internet Criminal Complaint Center), immediately stop contact with the scammer (you are potentially providing them more information which can be used to scam you), and learn how to protect yourself against these scams at Caution-http://www.ftc.gov < Caution-http://www.ftc.gov > (Federal Trade Commission's website)
-
How can I fill out Google's intern host matching form to optimize my chances of receiving a match?
I was selected for a summer internship 2016.I tried to be very open while filling the preference form: I choose many products as my favorite products and I said I'm open about the team I want to join.I even was very open in the location and start date to get host matching interviews (I negotiated the start date in the interview until both me and my host were happy.) You could ask your recruiter to review your form (there are very cool and could help you a lot since they have a bigger experience).Do a search on the potential team.Before the interviews, try to find smart question that you are going to ask for the potential host (do a search on the team to find nice and deep questions to impress your host). Prepare well your resume.You are very likely not going to get algorithm/data structure questions like in the first round. It's going to be just some friendly chat if you are lucky. If your potential team is working on something like machine learning, expect that they are going to ask you questions about machine learning, courses related to machine learning you have and relevant experience (projects, internship). Of course you have to study that before the interview. Take as long time as you need if you feel rusty. It takes some time to get ready for the host matching (it's less than the technical interview) but it's worth it of course.
-
How do I fill out the form of DU CIC? I couldn't find the link to fill out the form.
Just register on the admission portal and during registration you will get an option for the entrance based course. Just register there. There is no separate form for DU CIC.
-
How do you know if you need to fill out a 1099 form?
Assuming that you are talking about 1099-MISC. Note that there are other 1099s.check this post - Form 1099 MISC Rules & RegulationsQuick answer - A Form 1099 MISC must be filed for each person to whom payment is made of:$600 or more for services performed for a trade or business by people not treated as employees;Rent or prizes and awards that are not for service ($600 or more) and royalties ($10 or more);any fishing boat proceeds,gross proceeds of $600, or more paid to an attorney during the year, orWithheld any federal income tax under the backup withholding rules regardless of the amount of the payment, etc.
-
How can I make it easier for users to fill out a form on mobile apps?
I’ll tell you a secret - you can thank me later for this.If you want to make the form-filling experience easy for a user - make sure that you have a great UI to offer.Everything boils down to UI at the end.Axonator is one of the best mobile apps to collect data since it offers powerful features bundled with a simple UI.The problem with most of the mobile form apps is that they are overloaded with features that aren’t really necessary.The same doesn’t hold true for Axonator. It has useful features but it is very unlikely that the user will feel overwhelmed in using them.So, if you are inclined towards having greater form completion rates for your survey or any data collection projects, then Axonator is the way to go.Apart from that, there are other features that make the data collection process faster like offline data collection, rich data capture - audio, video, images, QR code & barcode data capture, live location & time capture, and more!Check all the features here!You will be able to complete more surveys - because productivity will certainly shoot up.Since you aren’t using paper forms, errors will drop signNowly.The cost of the paper & print will be saved - your office expenses will drop dramatically.No repeat work. No data entry. Time & money saved yet again.Analytics will empower you to make strategic decisions and explore new revenue opportunities.The app is dirt-cheap & you don’t any training to use the app. They come in with a smooth UI. Forget using, even creating forms for your apps is easy on the platform. Just drag & drop - and it’s ready for use. Anyone can build an app under hours.
-
When do I have to learn how to fill out a W-2 form?
Form W-2 is an obligatory form to be completed by every employer. Form W-2 doesn’t have to be filled out by the employee. It is given to inform the employee about the amount of his annual income and taxes withheld from it.You can find a lot of information here: http://bit.ly/2NjjlJi
Create this form in 5 minutes!
How to create an eSignature for the phs 2874 form
How to make an electronic signature for your Phs 2874 Form online
How to create an eSignature for your Phs 2874 Form in Chrome
How to create an electronic signature for signing the Phs 2874 Form in Gmail
How to create an eSignature for the Phs 2874 Form from your smartphone
How to generate an eSignature for the Phs 2874 Form on iOS devices
How to create an electronic signature for the Phs 2874 Form on Android OS
People also ask
-
What is Phs 2874 and how does it relate to airSlate SignNow?
Phs 2874 is a regulatory standard that ensures the security and compliance of electronic signatures. With airSlate SignNow, businesses can confidently send and eSign documents while adhering to Phs 2874 guidelines, making it a reliable choice for secure document management.
-
How does airSlate SignNow's pricing compare to other eSignature solutions regarding Phs 2874 compliance?
airSlate SignNow offers competitive pricing plans that align with the needs of businesses seeking Phs 2874 compliance. By choosing our solution, you not only gain access to cost-effective eSignature features but also ensure that your document practices meet industry standards.
-
What features does airSlate SignNow offer for users concerned about Phs 2874 compliance?
airSlate SignNow includes advanced features such as secure document storage, audit trails, and customizable workflows, all designed to support Phs 2874 compliance. These features not only enhance security but also streamline your document processes for better efficiency.
-
Can airSlate SignNow integrate with other platforms while maintaining Phs 2874 compliance?
Yes, airSlate SignNow offers seamless integrations with various applications, ensuring that your workflows remain efficient without compromising Phs 2874 compliance. Whether you use CRM systems or project management tools, our solution fits effortlessly into your existing ecosystem.
-
What are the benefits of using airSlate SignNow for businesses focused on Phs 2874 standards?
Businesses that prioritize Phs 2874 standards will find numerous benefits with airSlate SignNow, including enhanced security, reduced turnaround times for document signing, and improved client satisfaction. Our platform empowers you to manage documents with confidence, knowing you're compliant with industry regulations.
-
How user-friendly is airSlate SignNow for those needing to comply with Phs 2874?
airSlate SignNow is designed with user experience in mind, making it easy for anyone to send and eSign documents while complying with Phs 2874. Our intuitive interface ensures that both tech-savvy users and those less familiar with digital tools can navigate the platform effortlessly.
-
Is customer support available for users concerned with Phs 2874 compliance in airSlate SignNow?
Absolutely! airSlate SignNow provides robust customer support to assist users with any queries related to Phs 2874 compliance. Our dedicated support team is available to help you navigate compliance issues and optimize your document signing processes.
Get more for Phs 2874
Find out other Phs 2874
- How To eSign Hawaii Addressing Harassement
- How To eSign Arkansas Company Bonus Letter
- eSign Hawaii Promotion Announcement Secure
- eSign Alaska Worksheet Strengths and Weaknesses Myself
- How To eSign Rhode Island Overtime Authorization Form
- eSign Florida Payroll Deduction Authorization Safe
- eSign Delaware Termination of Employment Worksheet Safe
- Can I eSign New Jersey Job Description Form
- Can I eSign Hawaii Reference Checking Form
- Help Me With eSign Hawaii Acknowledgement Letter
- eSign Rhode Island Deed of Indemnity Template Secure
- eSign Illinois Car Lease Agreement Template Fast
- eSign Delaware Retainer Agreement Template Later
- eSign Arkansas Attorney Approval Simple
- eSign Maine Car Lease Agreement Template Later
- eSign Oregon Limited Power of Attorney Secure
- How Can I eSign Arizona Assignment of Shares
- How To eSign Hawaii Unlimited Power of Attorney
- How To eSign Louisiana Unlimited Power of Attorney
- eSign Oklahoma Unlimited Power of Attorney Now