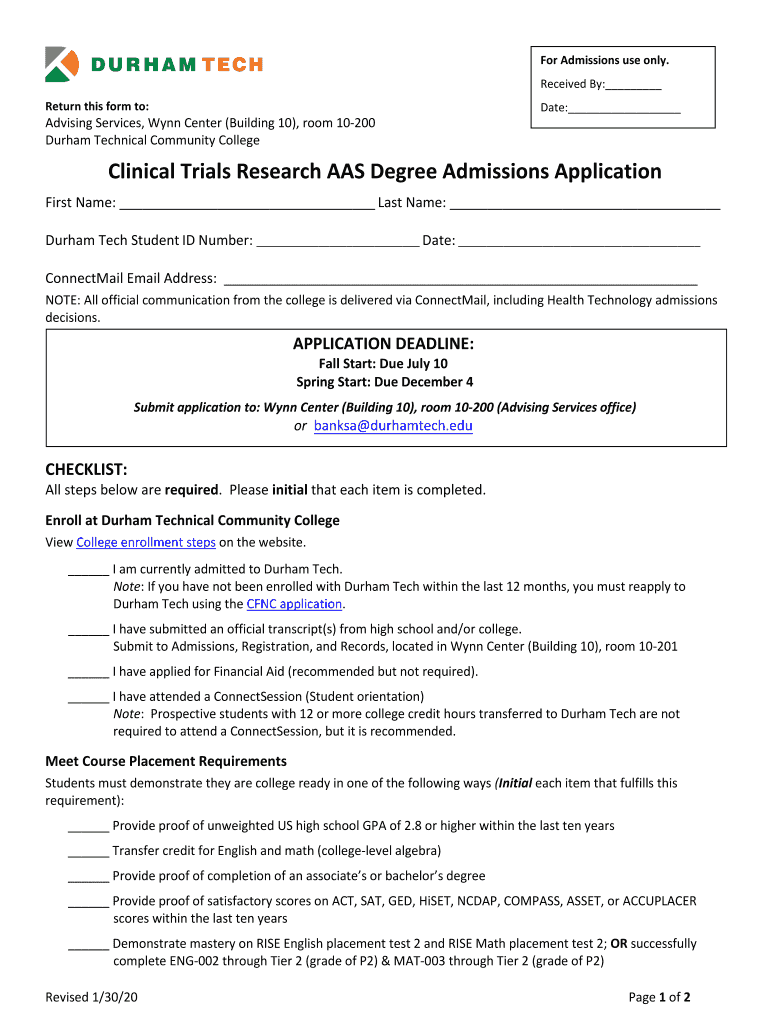
Clinical Trials Research AAS Degree Admissions Application Form


What is the Clinical Trials Research AAS Degree Admissions Application
The Clinical Trials Research AAS Degree Admissions Application is a formal document required for prospective students seeking admission into an Associate of Applied Science degree program focused on clinical trials research. This application is designed to collect essential information about the applicant, including educational background, work experience, and personal statements. The goal is to assess the applicant's qualifications and readiness for the program, which prepares students for careers in clinical research, data management, and regulatory affairs.
How to use the Clinical Trials Research AAS Degree Admissions Application
Using the Clinical Trials Research AAS Degree Admissions Application involves several steps to ensure that all required information is accurately provided. First, gather necessary documents such as transcripts, letters of recommendation, and a personal statement. Next, complete the application form by filling in personal details, educational history, and any relevant work experience. It is important to review the application thoroughly for accuracy before submission. Once completed, the application can be submitted electronically or via mail, depending on the institution's requirements.
Steps to complete the Clinical Trials Research AAS Degree Admissions Application
Completing the Clinical Trials Research AAS Degree Admissions Application involves a systematic approach:
- Gather required documents, including transcripts and recommendation letters.
- Fill out personal information, ensuring accuracy in contact details.
- Provide educational history, including institutions attended and degrees earned.
- Detail any relevant work experience in clinical research or related fields.
- Write a personal statement that reflects your interest in the program and career goals.
- Review the application for completeness and accuracy.
- Submit the application by the specified deadline, following the preferred submission method.
Legal use of the Clinical Trials Research AAS Degree Admissions Application
The legal use of the Clinical Trials Research AAS Degree Admissions Application is governed by various regulations that ensure the integrity and confidentiality of applicant information. Institutions must comply with federal and state laws regarding data protection, such as the Family Educational Rights and Privacy Act (FERPA). Additionally, ensuring that the application process is transparent and fair is essential to uphold the rights of applicants. Proper handling of personal information is crucial to maintain trust and comply with legal standards.
Required Documents
When applying for the Clinical Trials Research AAS Degree program, several documents are typically required to support the application. These may include:
- Official high school transcripts or equivalent.
- Transcripts from any post-secondary institutions attended.
- Letters of recommendation from educators or professionals in the field.
- A personal statement outlining your interest in clinical trials research.
- Any relevant certifications or licenses related to clinical research.
Eligibility Criteria
Eligibility criteria for the Clinical Trials Research AAS Degree Admissions Application may vary by institution but generally include the following:
- A high school diploma or equivalent.
- A minimum GPA requirement, often around 2.5 on a 4.0 scale.
- Completion of prerequisite courses, if applicable.
- Demonstrated interest in clinical trials research through relevant experience or coursework.
Quick guide on how to complete clinical trials research aas degree admissions application
Easily Prepare Clinical Trials Research AAS Degree Admissions Application on Any Device
Digital document management has gained popularity among organizations and individuals. It serves as an ideal eco-friendly substitute for traditional printed and signed paperwork, allowing you to obtain the appropriate form and securely save it online. airSlate SignNow provides you with all the tools necessary to create, modify, and electronically sign your documents promptly without delays. Manage Clinical Trials Research AAS Degree Admissions Application on any device with the airSlate SignNow apps for Android or iOS and enhance any document-related process today.
The Easiest Way to Modify and Electronically Sign Clinical Trials Research AAS Degree Admissions Application
- Locate Clinical Trials Research AAS Degree Admissions Application and click on Get Form to begin.
- Utilize the tools we provide to complete your form.
- Emphasize important sections of the documents or obscure sensitive details with tools specifically provided by airSlate SignNow for that purpose.
- Generate your electronic signature with the Sign tool, which takes mere seconds and holds the same legal validity as a conventional wet ink signature.
- Review all the details and click the Done button to save your modifications.
- Choose how you'd like to share your form, whether by email, SMS, invite link, or download it to your computer.
Eliminate concerns about lost or mislaid documents, tedious form searching, or errors that necessitate printing new document copies. airSlate SignNow fulfills your document management needs in just a few clicks from any device you prefer. Modify and electronically sign Clinical Trials Research AAS Degree Admissions Application and ensure excellent communication at every stage of the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the clinical trials research aas degree admissions application
How to make an eSignature for your PDF file online
How to make an eSignature for your PDF file in Google Chrome
The way to make an eSignature for signing PDFs in Gmail
The way to create an electronic signature from your mobile device
The best way to make an electronic signature for a PDF file on iOS
The way to create an electronic signature for a PDF file on Android devices
People also ask
-
What is the Clinical Trials Research AAS Degree Admissions Application process?
The Clinical Trials Research AAS Degree Admissions Application process involves several key steps, including submitting your completed application form, providing relevant transcripts, and meeting any additional program requirements. Ensure you check the specific deadlines and prerequisites for enrollment, as they can vary. This comprehensive approach will help streamline your admission into the program.
-
What are the key benefits of pursuing a Clinical Trials Research AAS Degree?
Pursuing a Clinical Trials Research AAS Degree provides numerous benefits, including developing essential skills for managing clinical research projects and enhancing your employability in a growing field. Graduates typically find opportunities in various industries, including pharmaceuticals and healthcare. This degree also gives you a competitive edge in the job market.
-
How much does the Clinical Trials Research AAS Degree Admissions Application cost?
The costs associated with the Clinical Trials Research AAS Degree Admissions Application can vary depending on the institution. Typically, there may be application fees, tuition rates, and additional expenses for materials and resources. It's essential to check with the specific school for detailed pricing information.
-
What features does the Clinical Trials Research AAS Degree program offer?
The Clinical Trials Research AAS Degree program includes features such as hands-on training, access to experienced faculty, and opportunities for internships in the clinical research field. Additionally, students may benefit from online resources and modern laboratory facilities. This comprehensive curriculum prepares students for real-world applications in clinical settings.
-
Are there any prerequisites for the Clinical Trials Research AAS Degree Admissions Application?
Yes, there are prerequisites for the Clinical Trials Research AAS Degree Admissions Application, which may include a high school diploma or equivalent, as well as specific coursework in science or mathematics. It is advisable to review the admission requirements of the institution you are interested in to ensure you meet all criteria before applying.
-
What types of careers can I pursue with a Clinical Trials Research AAS Degree?
Graduates with a Clinical Trials Research AAS Degree can pursue various careers in research administration, clinical trial coordination, data management, and regulatory affairs. With expertise in clinical trials, you can find positions in hospitals, pharmaceutical companies, and research institutions. This degree equips you with the necessary skills for a rewarding career in healthcare research.
-
How does the Clinical Trials Research AAS Degree program integrate with other fields?
The Clinical Trials Research AAS Degree program integrates with fields such as biotechnology, pharmacology, and healthcare management. This interdisciplinary approach enhances your understanding of how different sectors contribute to clinical research and trials. Collaborating across these fields broadens your career opportunities and professional network.
Get more for Clinical Trials Research AAS Degree Admissions Application
Find out other Clinical Trials Research AAS Degree Admissions Application
- How To Sign Wisconsin Real estate document
- Sign Montana Real estate investment proposal template Later
- How Do I Sign Washington Real estate investment proposal template
- Can I Sign Washington Real estate investment proposal template
- Sign Wisconsin Real estate investment proposal template Simple
- Can I Sign Kentucky Performance Contract
- How Do I Sign Florida Investment Contract
- Sign Colorado General Power of Attorney Template Simple
- How Do I Sign Florida General Power of Attorney Template
- Sign South Dakota Sponsorship Proposal Template Safe
- Sign West Virginia Sponsorship Proposal Template Free
- Sign Tennessee Investment Contract Safe
- Sign Maryland Consulting Agreement Template Fast
- Sign California Distributor Agreement Template Myself
- How Do I Sign Louisiana Startup Business Plan Template
- Can I Sign Nevada Startup Business Plan Template
- Sign Rhode Island Startup Business Plan Template Now
- How Can I Sign Connecticut Business Letter Template
- Sign Georgia Business Letter Template Easy
- Sign Massachusetts Business Letter Template Fast