Cleaning Validation Report Template Form


What is the Cleaning Validation Report Template
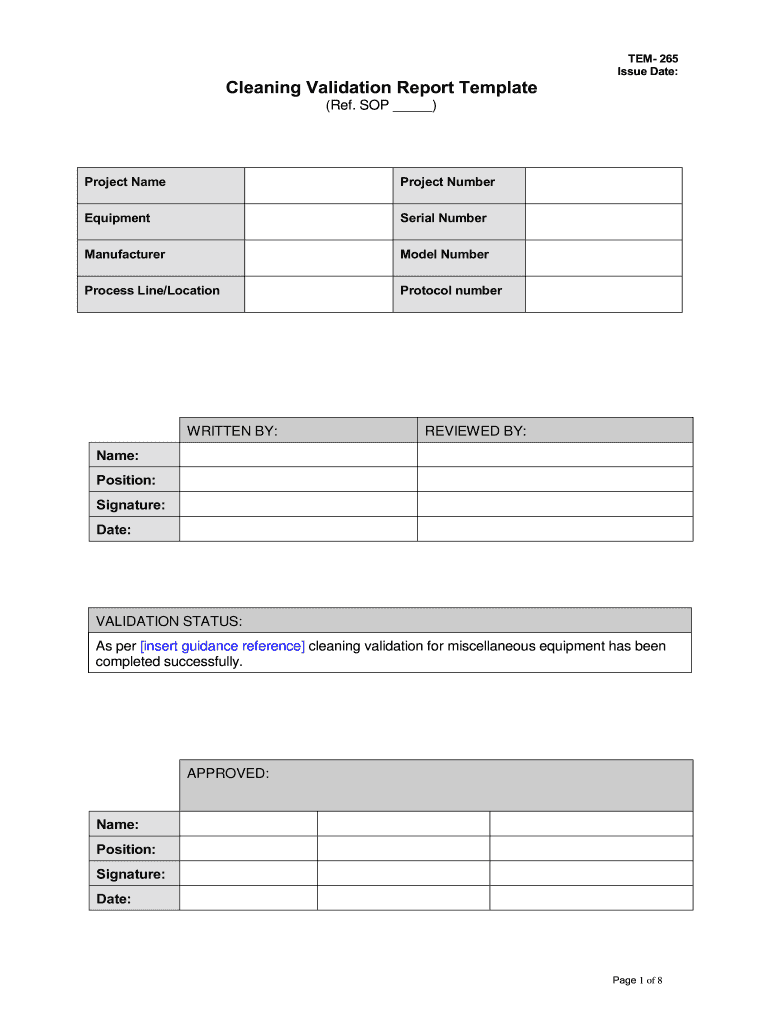
The cleaning validation report template is a structured document that outlines the procedures and results of cleaning validation processes in various industries, particularly in pharmaceuticals and biotechnology. This template serves as a formal record to demonstrate that cleaning processes effectively remove residues from equipment and surfaces, ensuring compliance with regulatory standards. It typically includes sections for objectives, methodology, results, and conclusions, making it essential for maintaining quality assurance and regulatory compliance.
How to Use the Cleaning Validation Report Template
Using the cleaning validation report template involves several key steps to ensure that all necessary information is captured accurately. First, gather all relevant data regarding the cleaning procedures performed, including the types of equipment cleaned, cleaning agents used, and the validation methods applied. Next, fill in the template with this information, ensuring that each section is completed thoroughly. It's important to include detailed results from any testing conducted to verify the effectiveness of the cleaning process. Finally, review the completed report for accuracy and compliance with regulatory requirements before submission.
Key Elements of the Cleaning Validation Report Template
The cleaning validation report template typically includes several crucial elements that must be addressed to ensure its effectiveness. These elements often consist of:
- Objective: A clear statement of the purpose of the cleaning validation.
- Scope: Details on the equipment and processes covered by the validation.
- Methodology: A description of the cleaning procedures and validation methods used.
- Results: Data and findings from the cleaning validation tests.
- Conclusion: An assessment of whether the cleaning process meets the required standards.
- Signatures: Signatures of responsible personnel to validate the report.
Steps to Complete the Cleaning Validation Report Template
Completing the cleaning validation report template involves a systematic approach to ensure all necessary information is accurately documented. The steps include:
- Identify the cleaning validation requirements specific to your industry and regulatory standards.
- Collect data from cleaning processes, including methods, agents, and equipment used.
- Fill out the template, ensuring each section is completed with precise and relevant information.
- Include results from any testing performed, such as residue analysis or microbial testing.
- Review the report for completeness and accuracy, ensuring compliance with applicable regulations.
- Obtain necessary signatures from authorized personnel to finalize the document.
Legal Use of the Cleaning Validation Report Template
The legal use of the cleaning validation report template is critical for organizations in regulated industries. To ensure that the report is legally valid, it must comply with relevant laws and regulations, such as those set forth by the FDA and other governing bodies. This includes maintaining proper documentation practices, ensuring that the report is signed by qualified personnel, and retaining records for a specified duration. Utilizing a reliable electronic signature solution can enhance the legal standing of the document, as it provides an audit trail and ensures compliance with eSignature laws.
Examples of Using the Cleaning Validation Report Template
Examples of using the cleaning validation report template can vary widely across industries. In pharmaceuticals, a company may use the template to validate the cleaning of a production line after a batch changeover. In biotechnology, the template can document the cleaning validation of equipment used in the manufacture of biologics. Each example highlights the importance of adhering to industry standards and regulatory requirements, ensuring that the cleaning processes are effective and that the products produced are safe for consumption.
Quick guide on how to complete cleaning validation report template
Effortlessly Prepare Cleaning Validation Report Template on Any Device
Digital document management has gained traction among businesses and individuals alike. It serves as an ideal environmentally friendly substitute for conventional printed and signed documents, as you can obtain the necessary form and securely preserve it online. airSlate SignNow equips you with all the resources required to create, amend, and electronically sign your documents swiftly without delays. Handle Cleaning Validation Report Template on any device using airSlate SignNow's Android or iOS applications and optimize any document-related process today.
How to Modify and Electronically Sign Cleaning Validation Report Template with Ease
- Locate Cleaning Validation Report Template and click on Get Form to begin.
- Utilize the tools we offer to complete your form.
- Emphasize relevant portions of your documents or obscure sensitive information with tools specifically provided by airSlate SignNow for that purpose.
- Create your electronic signature using the Sign feature, which takes mere seconds and holds the same legal validity as a traditional handwritten signature.
- Verify the details and click the Done button to save your changes.
- Choose your preferred method for sharing your form, whether by email, SMS, or invite link, or download it to your computer.
No more worrying about lost or misplaced files, tedious form navigation, or errors that require printing new document copies. airSlate SignNow meets your document management needs with just a few clicks from any device you choose. Edit and electronically sign Cleaning Validation Report Template to ensure excellent communication throughout your form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the cleaning validation report template
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is a cleaning validation report template?
A cleaning validation report template is a standardized document used to record the results of cleaning validation procedures in regulated industries. This template helps ensure compliance with industry standards and provides a clear, organized way to track validation activities.
-
Why do I need a cleaning validation report template?
Using a cleaning validation report template is essential for maintaining compliance with regulatory requirements. It helps streamline the documentation process, ensures consistency, and supports quality assurance efforts across your organization.
-
How can the airSlate SignNow platform assist with creating a cleaning validation report template?
The airSlate SignNow platform allows you to easily customize a cleaning validation report template to fit your specific needs. With its intuitive design and eSignature capabilities, you can create, send, and eSign documents seamlessly, saving time and improving efficiency.
-
Is the cleaning validation report template customizable?
Yes, the cleaning validation report template is fully customizable within the airSlate SignNow platform. You can modify fields, add your branding, and adjust the format to ensure it meets your unique requirements and regulatory standards.
-
What are the pricing options for using the cleaning validation report template in airSlate SignNow?
AirSlate SignNow offers various pricing plans suitable for different business needs. You can choose a plan that includes access to the cleaning validation report template, ensuring your documentation process is both efficient and cost-effective.
-
Can I integrate the cleaning validation report template with other software?
Absolutely! The airSlate SignNow platform supports integrations with many third-party applications, allowing you to seamlessly connect your cleaning validation report template with your existing tools and systems for enhanced workflow automation.
-
What are the benefits of using a cleaning validation report template?
Using a cleaning validation report template improves quality assurance and compliance by providing a structured format for documenting results. It also enhances accountability, streamlines communication, and reduces the risk of errors during validation processes.
Get more for Cleaning Validation Report Template
Find out other Cleaning Validation Report Template
- Can I Electronic signature Delaware Construction PDF
- How Can I Electronic signature Ohio Business Operations Document
- How Do I Electronic signature Iowa Construction Document
- How Can I Electronic signature South Carolina Charity PDF
- How Can I Electronic signature Oklahoma Doctors Document
- How Can I Electronic signature Alabama Finance & Tax Accounting Document
- How To Electronic signature Delaware Government Document
- Help Me With Electronic signature Indiana Education PDF
- How To Electronic signature Connecticut Government Document
- How To Electronic signature Georgia Government PDF
- Can I Electronic signature Iowa Education Form
- How To Electronic signature Idaho Government Presentation
- Help Me With Electronic signature Hawaii Finance & Tax Accounting Document
- How Can I Electronic signature Indiana Government PDF
- How Can I Electronic signature Illinois Finance & Tax Accounting PPT
- How To Electronic signature Maine Government Document
- How To Electronic signature Louisiana Education Presentation
- How Can I Electronic signature Massachusetts Government PDF
- How Do I Electronic signature Montana Government Document
- Help Me With Electronic signature Louisiana Finance & Tax Accounting Word