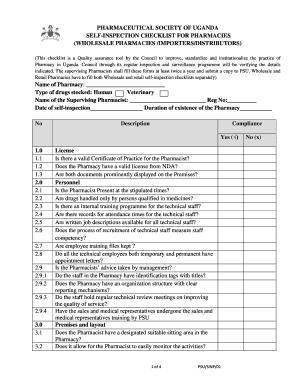
Self Inspection Checklist Pharmaceuticals Form


What is the self inspection checklist pharmaceuticals
The self inspection checklist pharmaceuticals is a comprehensive tool designed to help organizations in the pharmaceutical industry assess their compliance with Good Manufacturing Practices (GMP). This checklist serves as a guide for evaluating various operational aspects, including quality control, sanitation, and equipment maintenance. By utilizing this checklist, businesses can identify potential areas of non-compliance and implement corrective actions to ensure adherence to regulatory standards.
How to use the self inspection checklist pharmaceuticals
Using the self inspection checklist pharmaceuticals involves several key steps. First, gather all necessary documentation related to your operations, including previous inspection reports and standard operating procedures. Next, systematically go through each item on the checklist, evaluating your practices against the GMP requirements. It is essential to involve relevant team members in this process to gain diverse insights and ensure thoroughness. After completing the checklist, document any findings and develop an action plan to address identified issues.
Steps to complete the self inspection checklist pharmaceuticals
Completing the self inspection checklist pharmaceuticals can be broken down into clear steps:
- Assemble a team of qualified personnel to conduct the inspection.
- Review the checklist items to familiarize yourself with the GMP standards.
- Conduct a walkthrough of your facility, assessing each area against the checklist.
- Document findings, noting any areas of non-compliance or improvement opportunities.
- Prioritize issues based on severity and develop an action plan for resolution.
- Schedule follow-up inspections to ensure corrective actions have been implemented.
Legal use of the self inspection checklist pharmaceuticals
The legal use of the self inspection checklist pharmaceuticals hinges on its alignment with established regulations, such as those set forth by the FDA. For the checklist to be considered valid, it must accurately reflect the current GMP standards and be completed in a manner that can withstand regulatory scrutiny. This includes maintaining records of the inspection process, findings, and any corrective actions taken. Employing a reliable digital solution, such as signNow, can enhance the integrity of the documentation through secure eSignatures and audit trails.
Key elements of the self inspection checklist pharmaceuticals
Key elements of the self inspection checklist pharmaceuticals typically include:
- Quality management systems
- Personnel training and qualifications
- Facility and equipment maintenance
- Production processes and controls
- Laboratory controls and testing
- Record-keeping and documentation practices
Each of these elements plays a critical role in ensuring compliance with GMP and maintaining product quality and safety.
Examples of using the self inspection checklist pharmaceuticals
Examples of using the self inspection checklist pharmaceuticals can vary by organization. For instance, a pharmaceutical manufacturer might use the checklist to prepare for an upcoming FDA inspection, ensuring that all practices are compliant. A hospital pharmacy may utilize the checklist to evaluate its adherence to safety protocols and medication handling procedures. These practical applications demonstrate the checklist's versatility in promoting compliance and operational excellence across different sectors within the pharmaceutical industry.
Quick guide on how to complete self inspection checklist pharmaceuticals
Finish self inspection checklist pharmaceuticals effortlessly on any device
Web-based document management has become increasingly popular among businesses and individuals. It offers a great eco-friendly substitute for traditional printed and signed documents, allowing you to obtain the necessary form and securely store it online. airSlate SignNow equips you with all the tools required to create, modify, and eSign your documents swiftly without any hold-ups. Manage gmp self inspection checklist on any device using airSlate SignNow's Android or iOS applications and enhance any document-centric workflow today.
The easiest way to modify and eSign self inspection in pharmaceutical industry with ease
- Locate gmp self inspection checklist for food industry and click on Get Form to begin.
- Make use of the tools we provide to complete your document.
- Emphasize important sections of your documents or obscure sensitive information with tools specifically provided by airSlate SignNow for that purpose.
- Generate your eSignature using the Sign tool, which takes mere seconds and holds the same legal validity as a conventional wet ink signature.
- Review all the information carefully and click on the Done button to save your modifications.
- Select your preferred method of sending your form, whether by email, text message (SMS), invitation link, or download it to your computer.
Forget about lost or misplaced documents, tiring form searches, or mistakes that necessitate printing new copies. airSlate SignNow addresses all your document management needs in just a few clicks from any device you prefer. Edit and eSign self inspection checklist in pharmaceutical industry while ensuring excellent communication at every stage of the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Related searches to gmp self inspection checklist for food industry
Create this form in 5 minutes!
How to create an eSignature for the self inspection checklist in pharmaceutical industry
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask hospital pharmacy inspection checklist
-
What is a self inspection checklist pharmaceuticals?
A self inspection checklist pharmaceuticals is a structured tool that helps organizations in the pharmaceutical industry assess their compliance with regulatory standards. This checklist enables businesses to review their processes and identify areas that require improvement, ensuring adherence to quality and safety regulations.
-
How can airSlate SignNow help with my self inspection checklist pharmaceuticals?
airSlate SignNow offers an efficient platform to create, manage, and sign self inspection checklists pharmaceuticals electronically. This solution simplifies document workflows, ensuring your team can easily collaborate and maintain compliance records securely.
-
What features should I look for in a self inspection checklist pharmaceuticals tool?
When seeking a self inspection checklist pharmaceuticals tool, prioritize features like customizable templates, electronic signatures, audit trail functionality, and integration capabilities with other systems. airSlate SignNow embodies these qualities, providing a comprehensive solution to streamline your inspection process.
-
Is airSlate SignNow cost-effective for managing self inspection checklist pharmaceuticals?
Yes, airSlate SignNow offers a cost-effective solution for managing your self inspection checklist pharmaceuticals. With flexible pricing plans, businesses, regardless of their size, can leverage powerful features without overspending, ultimately resulting in better compliance management.
-
Can I integrate airSlate SignNow with other software for my self inspection checklist pharmaceuticals?
Absolutely! airSlate SignNow integrates seamlessly with various software solutions, enhancing your self inspection checklist pharmaceuticals process. Whether it's CRM systems or document management tools, integration capabilities enable you to streamline workflows and enhance productivity.
-
What are the benefits of using airSlate SignNow for a self inspection checklist pharmaceuticals?
Using airSlate SignNow for your self inspection checklist pharmaceuticals provides numerous benefits, such as increased efficiency, reduced paper usage, and improved accuracy. The electronic signature feature allows for quicker approvals and better document tracking, ensuring compliance standards are met.
-
Is it easy to use airSlate SignNow for creating a self inspection checklist pharmaceuticals?
Yes, airSlate SignNow is designed with an intuitive interface that makes it easy for users to create a self inspection checklist pharmaceuticals. You don't need technical expertise; the platform guides users through the process, allowing for quick and straightforward checklist generation.
Get more for gmp self inspection checklist
Find out other self inspection in pharmaceutical industry
- Sign Nevada Non-Profit LLC Operating Agreement Free
- Sign Non-Profit Document New Mexico Mobile
- Sign Alaska Orthodontists Business Plan Template Free
- Sign North Carolina Life Sciences Purchase Order Template Computer
- Sign Ohio Non-Profit LLC Operating Agreement Secure
- Can I Sign Ohio Non-Profit LLC Operating Agreement
- Sign South Dakota Non-Profit Business Plan Template Myself
- Sign Rhode Island Non-Profit Residential Lease Agreement Computer
- Sign South Carolina Non-Profit Promissory Note Template Mobile
- Sign South Carolina Non-Profit Lease Agreement Template Online
- Sign Oregon Life Sciences LLC Operating Agreement Online
- Sign Texas Non-Profit LLC Operating Agreement Online
- Can I Sign Colorado Orthodontists Month To Month Lease
- How Do I Sign Utah Non-Profit Warranty Deed
- Help Me With Sign Colorado Orthodontists Purchase Order Template
- Sign Virginia Non-Profit Living Will Fast
- How To Sign Virginia Non-Profit Lease Agreement Template
- How To Sign Wyoming Non-Profit Business Plan Template
- How To Sign Wyoming Non-Profit Credit Memo
- Sign Wisconsin Non-Profit Rental Lease Agreement Simple