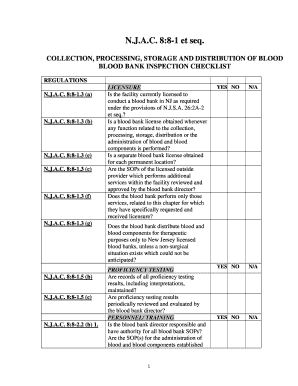
Fda Blood Bank Inspection Checklist Form


What is the FDA Blood Bank Inspection Checklist
The FDA blood bank inspection checklist is a comprehensive tool designed to ensure that blood banks comply with federal regulations and maintain high standards of safety and quality. This checklist outlines the essential criteria that blood banks must meet during inspections, covering areas such as facility cleanliness, equipment maintenance, and staff training. By adhering to this checklist, blood banks can demonstrate their commitment to safeguarding public health and ensuring the safety of blood donations.
How to Use the FDA Blood Bank Inspection Checklist
Utilizing the FDA blood bank inspection checklist involves a systematic approach to ensure that all necessary criteria are met before an inspection. Start by reviewing each item on the checklist thoroughly. Assign responsibilities to staff members for each section, ensuring that everyone understands their role in maintaining compliance. Regularly update the checklist based on any changes in regulations or operational procedures. Conduct internal audits using the checklist to identify areas for improvement and to prepare for official inspections.
Key Elements of the FDA Blood Bank Inspection Checklist
The key elements of the FDA blood bank inspection checklist include various compliance categories such as:
- Facility Standards: Ensuring the physical environment is clean and well-maintained.
- Equipment Calibration: Regular checks and maintenance of equipment used in blood collection and testing.
- Staff Training: Verification that all personnel are adequately trained in safety protocols and operational procedures.
- Donor Safety: Procedures to ensure the safety and confidentiality of blood donors.
- Record Keeping: Accurate documentation of all processes related to blood collection and testing.
Steps to Complete the FDA Blood Bank Inspection Checklist
Completing the FDA blood bank inspection checklist involves several steps:
- Gather all relevant documents and records related to blood bank operations.
- Review each item on the checklist, ensuring that all criteria are met.
- Document any areas of non-compliance and develop a plan for addressing these issues.
- Conduct a mock inspection to simulate the actual process and identify any potential gaps.
- Finalize the checklist and ensure all staff are informed of their responsibilities.
Legal Use of the FDA Blood Bank Inspection Checklist
The legal use of the FDA blood bank inspection checklist is crucial for compliance with federal regulations. It serves as a formal document that can be referenced during inspections and audits. By maintaining accurate records of the checklist's completion, blood banks can demonstrate their adherence to legal standards. This documentation is essential not only for regulatory compliance but also for protecting the organization in case of legal inquiries or disputes regarding blood safety practices.
Examples of Using the FDA Blood Bank Inspection Checklist
Examples of using the FDA blood bank inspection checklist include:
- Conducting quarterly internal audits to assess compliance with safety standards.
- Preparing for an upcoming FDA inspection by ensuring all checklist items are addressed.
- Training new staff members on the importance of compliance and how to utilize the checklist effectively.
Quick guide on how to complete fda blood bank inspection checklist
Finish Fda Blood Bank Inspection Checklist effortlessly on any gadget
Digital document management has become increasingly popular among businesses and individuals alike. It offers an ideal eco-friendly substitute for traditional printed and signed documents, as you can easily access the right form and securely store it online. airSlate SignNow provides you with all the tools necessary to create, edit, and eSign your documents promptly without any delays. Manage Fda Blood Bank Inspection Checklist on any device with airSlate SignNow's Android or iOS applications and enhance any document-oriented process today.
The simplest method to edit and eSign Fda Blood Bank Inspection Checklist without hassle
- Locate Fda Blood Bank Inspection Checklist and click on Get Form to begin.
- Utilize the tools we provide to complete your document.
- Highlight pertinent sections of your documents or obscure sensitive information with tools that airSlate SignNow offers specifically for that purpose.
- Create your eSignature using the Sign feature, which takes seconds and holds the same legal significance as a conventional wet ink signature.
- Review all the information and click on the Done button to save your changes.
- Select how you wish to send your form, via email, text message (SMS), or invitation link, or download it to your computer.
Say goodbye to lost or misplaced documents, tedious form searches, or mistakes that necessitate printing new document copies. airSlate SignNow meets your document management needs in just a few clicks from any device you choose. Edit and eSign Fda Blood Bank Inspection Checklist and ensure outstanding communication at any stage of your form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the fda blood bank inspection checklist
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is a bank inspection checklist?
A bank inspection checklist is a structured list of items and standards that need to be reviewed during a bank's operational inspection. It helps financial institutions ensure compliance with regulations and internal policies, facilitating a thorough review process for audits.
-
How can airSlate SignNow assist with my bank inspection checklist?
airSlate SignNow provides an efficient platform for creating, sending, and eSigning your bank inspection checklist. It simplifies document management, allowing your team to collaborate in real-time, ensuring that all necessary checks are completed and documented properly.
-
Is airSlate SignNow a cost-effective solution for bank inspection checklists?
Yes, airSlate SignNow is a cost-effective solution for managing bank inspection checklists. With affordable pricing plans and features that enhance productivity, it helps banks streamline their inspection processes without breaking the budget.
-
What features does airSlate SignNow offer for bank inspection checklists?
airSlate SignNow offers features such as customizable templates for bank inspection checklists, electronic signatures, document tracking, and storage. These functionalities ensure that all inspection documents are easily accessible and updatable.
-
Can I integrate airSlate SignNow with my existing systems for bank inspections?
Absolutely! airSlate SignNow allows seamless integration with various systems, including CRM and project management tools. This ensures that your bank inspection checklist workflow aligns with your existing business operations.
-
What are the benefits of using an electronic bank inspection checklist?
Using an electronic bank inspection checklist streamlines the inspection process, enhances collaboration, and minimizes paperwork. It allows for quicker turnaround times, easier tracking, and compliance with regulatory standards.
-
How secure is airSlate SignNow for managing bank inspection checklists?
airSlate SignNow ensures high-level security for all documents, including bank inspection checklists. It employs robust encryption and secure access measures to protect sensitive information during the inspection process.
Get more for Fda Blood Bank Inspection Checklist
- Wine club sign up form
- Receipt of certificate form
- Pony club medical armband form
- Sarvoday bank mehsana form
- Golf cart rental agreement amp form
- Rowlett police department alarm permit application form
- Rowlett police departmentalarm permit application form
- W va code r 48 3 2 criteria for health and safety form
Find out other Fda Blood Bank Inspection Checklist
- Sign Ohio Sports LLC Operating Agreement Easy
- Sign New Jersey Real Estate Limited Power Of Attorney Computer
- Sign New Mexico Real Estate Contract Safe
- How To Sign South Carolina Sports Lease Termination Letter
- How Can I Sign New York Real Estate Memorandum Of Understanding
- Sign Texas Sports Promissory Note Template Online
- Sign Oregon Orthodontists Last Will And Testament Free
- Sign Washington Sports Last Will And Testament Free
- How Can I Sign Ohio Real Estate LLC Operating Agreement
- Sign Ohio Real Estate Quitclaim Deed Later
- How Do I Sign Wisconsin Sports Forbearance Agreement
- How To Sign Oregon Real Estate Resignation Letter
- Can I Sign Oregon Real Estate Forbearance Agreement
- Sign Pennsylvania Real Estate Quitclaim Deed Computer
- How Do I Sign Pennsylvania Real Estate Quitclaim Deed
- How Can I Sign South Dakota Orthodontists Agreement
- Sign Police PPT Alaska Online
- How To Sign Rhode Island Real Estate LLC Operating Agreement
- How Do I Sign Arizona Police Resignation Letter
- Sign Texas Orthodontists Business Plan Template Later