Form 725 Raw Material Evaluation Form Pharmaceutical Quality


What is the Form 725 Raw Material Evaluation Form Pharmaceutical Quality
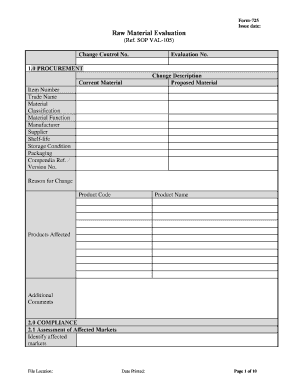
The Form 725 Raw Material Evaluation Form Pharmaceutical Quality is a specialized document used in the pharmaceutical industry to assess the quality of raw materials before they are utilized in production. This form ensures that all materials meet stringent quality standards, which is essential for maintaining safety and efficacy in pharmaceutical products. It includes sections for documenting the source of materials, specifications, testing results, and compliance with regulatory requirements.
How to use the Form 725 Raw Material Evaluation Form Pharmaceutical Quality
Using the Form 725 involves several key steps to ensure accurate and compliant evaluation of raw materials. First, gather all necessary information about the raw material, including its origin, supplier details, and any relevant testing data. Next, complete the form by filling in all required fields, ensuring that each section is clear and precise. After completing the form, it should be reviewed by a qualified professional to confirm that all information is accurate and meets regulatory standards before submission.
Key elements of the Form 725 Raw Material Evaluation Form Pharmaceutical Quality
Important elements of the Form 725 include:
- Material Identification: Details about the raw material, including its name, code, and description.
- Supplier Information: Contact details and background information about the supplier.
- Quality Specifications: Standards that the raw material must meet, including physical and chemical properties.
- Testing Results: Documentation of any tests performed on the raw material, along with the results.
- Compliance Statements: Affirmations that the material meets all relevant regulatory requirements.
Steps to complete the Form 725 Raw Material Evaluation Form Pharmaceutical Quality
Completing the Form 725 involves a systematic approach:
- Collect all necessary data about the raw material.
- Fill out the identification section with accurate details.
- Document supplier information thoroughly.
- Enter quality specifications and ensure they align with industry standards.
- Include testing results, ensuring they are from accredited laboratories.
- Review the completed form for accuracy and completeness.
- Submit the form to the relevant authority or department for approval.
Legal use of the Form 725 Raw Material Evaluation Form Pharmaceutical Quality
The legal use of the Form 725 is crucial for compliance with industry regulations. The form must be completed accurately to ensure that the raw materials are suitable for pharmaceutical production. Failure to adhere to legal standards can result in penalties, including fines or product recalls. It is essential to maintain records of the completed forms for auditing purposes and to demonstrate compliance with Good Manufacturing Practices (GMP).
Examples of using the Form 725 Raw Material Evaluation Form Pharmaceutical Quality
Examples of the Form 725 in action include:
- Pharmaceutical companies using the form to evaluate active pharmaceutical ingredients (APIs) before production.
- Contract manufacturers utilizing the form to verify the quality of raw materials sourced from suppliers.
- Quality assurance teams employing the form to document compliance during internal audits.
Quick guide on how to complete form 725 raw material evaluation form pharmaceutical quality
Effortlessly Prepare Form 725 Raw Material Evaluation Form Pharmaceutical Quality on Any Device
Managing documents online has gained popularity among businesses and individuals alike. It offers an excellent eco-friendly substitute for traditional printed and signed documents, as you can easily find the right form and securely store it online. airSlate SignNow provides all the tools necessary to swiftly create, modify, and eSign your documents without delay. Handle Form 725 Raw Material Evaluation Form Pharmaceutical Quality on any device using the airSlate SignNow Android or iOS applications and simplify any document-related processes today.
How to Modify and eSign Form 725 Raw Material Evaluation Form Pharmaceutical Quality with Ease
- Obtain Form 725 Raw Material Evaluation Form Pharmaceutical Quality and click Get Form to begin.
- Utilize the tools we offer to complete your document.
- Highlight important sections of the documents or obscure sensitive information with the tools that airSlate SignNow offers specifically for this purpose.
- Create your signature with the Sign tool, which takes just seconds and has the same legal validity as a traditional handwritten signature.
- Review the details and click the Done button to save your updates.
- Select your preferred method for sharing your form, such as via email, SMS, or an invite link, or download it to your computer.
Eliminate concerns about lost or misplaced files, tedious form searching, or errors that necessitate printing new document copies. airSlate SignNow addresses all your document management needs in just a few clicks from your chosen device. Alter and eSign Form 725 Raw Material Evaluation Form Pharmaceutical Quality to ensure excellent communication at every stage of the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the form 725 raw material evaluation form pharmaceutical quality
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is raw material evaluation in the context of airSlate SignNow?
Raw material evaluation refers to the process of assessing the quality and suitability of materials before they are used in production. With airSlate SignNow, businesses can streamline their raw material evaluation processes by automating documentation and eSigning, ensuring that all evaluations are properly recorded and easily accessible.
-
How does airSlate SignNow enhance the raw material evaluation process?
AirSlate SignNow enhances the raw material evaluation process by providing a user-friendly platform where teams can collaborate and electronically sign necessary documents in real-time. This not only speeds up the evaluation process but also reduces errors associated with manual paperwork, allowing for more efficient decision-making.
-
What are the pricing options for airSlate SignNow's raw material evaluation tools?
AirSlate SignNow offers various pricing plans designed to fit businesses of all sizes. Each plan includes features that support raw material evaluation, allowing companies to choose the level of functionality that best meets their needs, from basic document signing to advanced workflow automation.
-
Can airSlate SignNow integrate with other tools for raw material evaluation?
Yes, airSlate SignNow seamlessly integrates with numerous third-party applications that are commonly used in raw material evaluation. These integrations facilitate a more holistic approach to handling documents related to evaluations, ensuring that all data is synchronized across platforms.
-
What are the key benefits of using airSlate SignNow for raw material evaluation?
The key benefits of using airSlate SignNow for raw material evaluation include improved efficiency, reduced processing time, and enhanced compliance. By automating document management and eSigning, businesses can focus more on evaluating quality rather than being bogged down by administrative tasks.
-
Is airSlate SignNow suitable for small businesses conducting raw material evaluations?
Absolutely! AirSlate SignNow is designed to be cost-effective and user-friendly, making it an ideal solution for small businesses conducting raw material evaluations. The platform’s scalable features allow small teams to manage their evaluation processes without the need for extensive resources.
-
How secure is the raw material evaluation documentation in airSlate SignNow?
AirSlate SignNow takes document security seriously, employing advanced encryption and compliance standards to protect your raw material evaluation documentation. This ensures that sensitive information remains confidential and secure, providing peace of mind for businesses.
Get more for Form 725 Raw Material Evaluation Form Pharmaceutical Quality
- Renewal form occupation tax certificate cherokee county
- Florida personal auto insurance form
- Nevada probate forms
- Broward county public schools school asthma action plan form
- Student enrollment form schools cms k12 nc
- Maths key skills stage 4 477977267 form
- Application for authorization and statutory declaration form
- Settlement between two parties agreement template form
Find out other Form 725 Raw Material Evaluation Form Pharmaceutical Quality
- eSign Texas Rental lease agreement Mobile
- eSign Utah Rental agreement lease Easy
- How Can I eSign North Dakota Rental lease agreement forms
- eSign Rhode Island Rental lease agreement forms Now
- eSign Georgia Rental lease agreement template Simple
- Can I eSign Wyoming Rental lease agreement forms
- eSign New Hampshire Rental lease agreement template Online
- eSign Utah Rental lease contract Free
- eSign Tennessee Rental lease agreement template Online
- eSign Tennessee Rental lease agreement template Myself
- eSign West Virginia Rental lease agreement template Safe
- How To eSign California Residential lease agreement form
- How To eSign Rhode Island Residential lease agreement form
- Can I eSign Pennsylvania Residential lease agreement form
- eSign Texas Residential lease agreement form Easy
- eSign Florida Residential lease agreement Easy
- eSign Hawaii Residential lease agreement Online
- Can I eSign Hawaii Residential lease agreement
- eSign Minnesota Residential lease agreement Simple
- How To eSign Pennsylvania Residential lease agreement