Fda Target Product Profile Template Form


What is the FDA target product profile template?
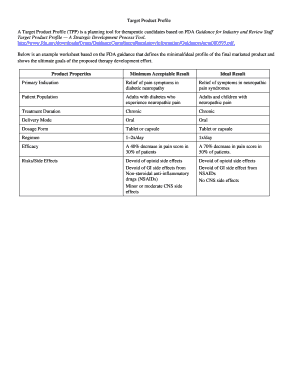
The FDA target product profile template is a structured document that outlines the desired characteristics and specifications of a product intended for regulatory submission. This template serves as a roadmap for developers and researchers, helping them articulate the product's intended use, target population, and key attributes. It is particularly important in the pharmaceutical and biotechnology sectors, where clear communication of product goals is essential for successful regulatory approval. By detailing aspects such as efficacy, safety, and quality, the template aids in aligning development efforts with regulatory expectations.
Key elements of the FDA target product profile template
Several critical components make up the FDA target product profile template. These elements include:
- Product description: A comprehensive overview of the product, including its formulation and mechanism of action.
- Indications for use: Specific conditions or diseases the product aims to treat or prevent.
- Target population: Characteristics of the patient population, such as age, gender, and disease stage.
- Efficacy endpoints: The primary and secondary outcomes used to measure the product's effectiveness.
- Safety profile: Anticipated adverse effects and safety considerations relevant to the product.
- Regulatory considerations: Key regulatory pathways and requirements for approval.
Steps to complete the FDA target product profile template
Completing the FDA target product profile template involves several systematic steps:
- Gather relevant data: Collect all necessary information regarding the product, including research findings and clinical data.
- Define the product's purpose: Clearly articulate the intended use and target population for the product.
- Identify key attributes: Outline the critical characteristics that the product must possess to meet regulatory requirements.
- Draft the template: Fill in the template with the gathered information, ensuring clarity and precision.
- Review and revise: Conduct a thorough review of the completed template, making necessary adjustments for accuracy and completeness.
- Seek feedback: Share the draft with stakeholders for input and suggestions before finalizing.
Legal use of the FDA target product profile template
The legal use of the FDA target product profile template is crucial for ensuring compliance with regulatory standards. When utilizing the template, it is essential to adhere to the guidelines set forth by the FDA, which include:
- Accuracy: All information provided must be truthful and supported by scientific evidence.
- Confidentiality: Sensitive data should be handled according to applicable privacy laws and regulations.
- Timeliness: Submissions should be made within the specified timeframes to avoid delays in the approval process.
By following these legal guidelines, developers can enhance the credibility of their submissions and facilitate a smoother regulatory review process.
How to obtain the FDA target product profile template
The FDA target product profile template can typically be obtained through the FDA's official website or by contacting the relevant regulatory division. The template is often available in downloadable formats, allowing users to customize it according to their specific product needs. Additionally, industry associations and regulatory consultants may provide resources and guidance on accessing and utilizing the template effectively. It is advisable to ensure that the most current version of the template is used, as regulatory requirements may evolve over time.
Examples of using the FDA target product profile template
Utilizing the FDA target product profile template can take various forms, depending on the product and its intended use. For instance:
- A pharmaceutical company may use the template to outline a new drug's intended indications and efficacy endpoints before initiating clinical trials.
- A biotechnology firm might employ the template to define the target population and safety profile for a novel therapeutic product.
- Researchers developing a medical device can leverage the template to articulate the device's purpose and regulatory pathway, ensuring alignment with FDA expectations.
These examples illustrate how the template serves as a foundational tool in the product development process, facilitating communication and regulatory compliance.
Quick guide on how to complete fda target product profile template
Effortlessly Prepare Fda Target Product Profile Template on Any Device
Digital document management has gained traction among businesses and individuals. It offers an excellent environmentally friendly alternative to conventional printed and signed documents, allowing you to access the right form and securely store it online. airSlate SignNow equips you with all the necessary tools to create, modify, and eSign your documents quickly and without interruptions. Manage Fda Target Product Profile Template on any platform using the airSlate SignNow Android or iOS applications and streamline any document-related task today.
The simplest way to modify and eSign Fda Target Product Profile Template effortlessly
- Obtain Fda Target Product Profile Template and select Get Form to commence.
- Utilize the tools we offer to complete your form.
- Emphasize pertinent sections of your documents or redact sensitive information with tools specifically designed by airSlate SignNow for that purpose.
- Generate your eSignature using the Sign tool, which takes just seconds and holds the same legal validity as a standard pen-and-ink signature.
- Verify all details and click on the Done button to save your modifications.
- Choose your preferred method to send your form, whether by email, SMS, invitation link, or download it to your computer.
Say goodbye to lost or misplaced documents, tedious form searching, or mistakes that necessitate printing new document copies. airSlate SignNow meets all your document management requirements with just a few clicks from any device of your choice. Modify and eSign Fda Target Product Profile Template to ensure excellent communication throughout every stage of the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the fda target product profile template
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is a target product profile template?
A target product profile template is a strategic tool designed to outline the key elements and outcomes of a product. It helps businesses visualize their product's potential in the market and define its main attributes, benefits, and target audience.
-
How can a target product profile template benefit my business?
Using a target product profile template can streamline the product development process by providing clarity and direction. It allows teams to align on objectives, prioritize features, and enhance overall product strategy, ultimately leading to improved market success.
-
Is there a cost associated with using the target product profile template in airSlate SignNow?
While airSlate SignNow offers a variety of features, using the target product profile template is included in our subscription plans. Our cost-effective solution ensures you get access to all essential tools without hidden fees.
-
What features are included in the airSlate SignNow target product profile template?
The airSlate SignNow target product profile template includes customizable sections for product details, target markets, competitive analysis, and preferred features. This makes it easy to tailor the template to your specific product and business needs.
-
Can the target product profile template integrate with other tools?
Yes, the airSlate SignNow target product profile template can seamlessly integrate with various project management and collaboration tools. This enhances workflow efficiency and allows for better communication among teams working on product development.
-
How do I get started with the target product profile template on airSlate SignNow?
Getting started is simple! Sign up for an account on airSlate SignNow, navigate to the templates section, and select the target product profile template. You'll be able to customize it to meet your specific product needs right away.
-
What industries can benefit from the target product profile template?
The target product profile template is versatile and can benefit a wide range of industries, including healthcare, technology, and consumer goods. Any business looking to improve its product strategy can leverage this template to gain insights and focus their efforts.
Get more for Fda Target Product Profile Template
- Form 15 sample application for membership from a community
- U100 formular
- Aviva change of address form
- Zak2 doc form
- Methods of requesting a transcript university of houston victoria form
- Request for supervisory approval to travel on official university business form
- Bachelor of social work program fayetteville state university form
- Fillable online transcript verification form fax
Find out other Fda Target Product Profile Template
- Sign New Mexico Real Estate Contract Safe
- How To Sign South Carolina Sports Lease Termination Letter
- How Can I Sign New York Real Estate Memorandum Of Understanding
- Sign Texas Sports Promissory Note Template Online
- Sign Oregon Orthodontists Last Will And Testament Free
- Sign Washington Sports Last Will And Testament Free
- How Can I Sign Ohio Real Estate LLC Operating Agreement
- Sign Ohio Real Estate Quitclaim Deed Later
- How Do I Sign Wisconsin Sports Forbearance Agreement
- How To Sign Oregon Real Estate Resignation Letter
- Can I Sign Oregon Real Estate Forbearance Agreement
- Sign Pennsylvania Real Estate Quitclaim Deed Computer
- How Do I Sign Pennsylvania Real Estate Quitclaim Deed
- How Can I Sign South Dakota Orthodontists Agreement
- Sign Police PPT Alaska Online
- How To Sign Rhode Island Real Estate LLC Operating Agreement
- How Do I Sign Arizona Police Resignation Letter
- Sign Texas Orthodontists Business Plan Template Later
- How Do I Sign Tennessee Real Estate Warranty Deed
- Sign Tennessee Real Estate Last Will And Testament Free