Form 1572 2016-2026


What is the Form 1572
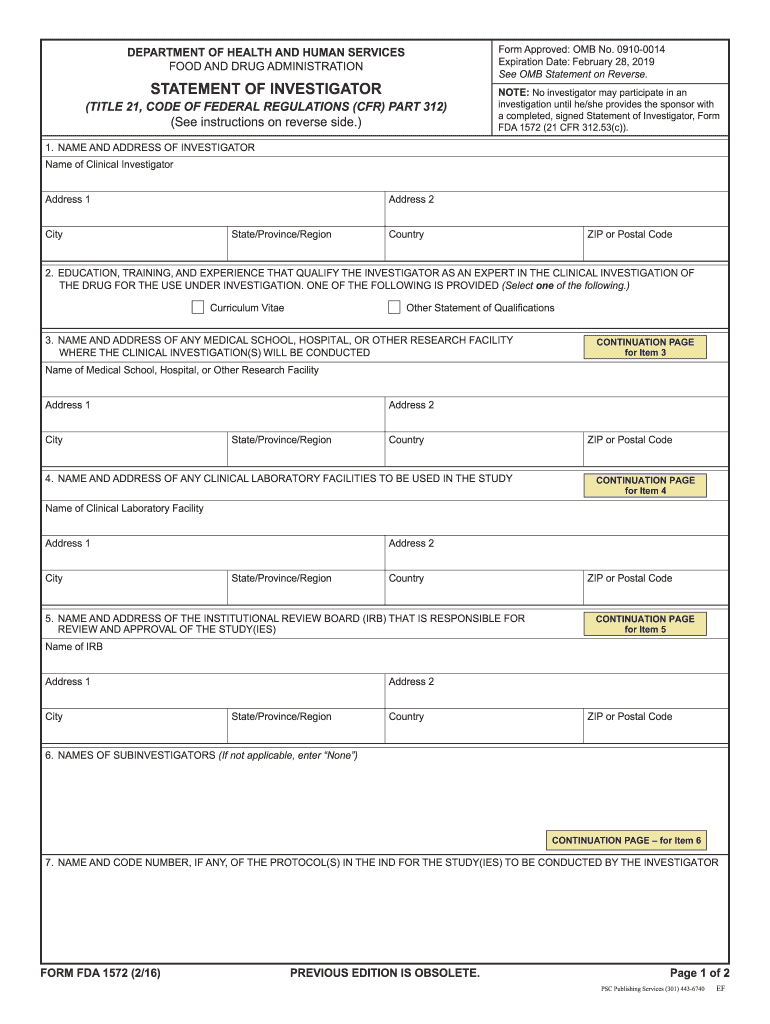
The Form 1572, also known as the Investigator's Brochure, is a critical document used primarily in clinical research. It serves as a comprehensive summary of the clinical trial protocol, detailing the investigational product, study design, and the responsibilities of the investigator. This form is essential for ensuring that all parties involved in the research comply with regulatory requirements and ethical standards. It includes vital information necessary for the safe and effective conduct of clinical trials.
How to complete the Form 1572
Completing the Form 1572 requires careful attention to detail. Start by gathering all necessary information, including the names and contact details of the principal investigator and any sub-investigators. Ensure that you include the study title, the investigational product, and the protocol number. It is crucial to review the required fields thoroughly and attach any supplementary documents as needed. After filling out the form, double-check for accuracy before submission to avoid delays in the approval process.
Key elements of the Form 1572
The Form 1572 includes several key elements that are essential for its validity. These elements consist of the investigator's qualifications, the institution's information, and a detailed description of the investigational product. Additionally, it outlines the responsibilities of the investigator, including compliance with Good Clinical Practice (GCP) guidelines. Each section must be completed accurately to ensure the form meets the necessary regulatory standards.
Steps to obtain the Form 1572
Obtaining the Form 1572 is a straightforward process. Researchers can typically download the form from the appropriate regulatory agency's website or request it directly from the institution conducting the clinical trial. Ensure that you are using the most current version of the form to avoid issues with outdated information. If assistance is needed, consider reaching out to the regulatory affairs department of your institution for guidance.
Legal use of the Form 1572
The legal use of the Form 1572 is governed by federal regulations and guidelines. It is essential for investigators to understand that this form must be submitted to the Food and Drug Administration (FDA) as part of the Investigational New Drug (IND) application process. Failure to comply with the legal requirements associated with the Form 1572 can result in penalties, including the rejection of the clinical trial application or legal repercussions for the investigator and the institution.
Form Submission Methods
Submitting the Form 1572 can be done through various methods, including online submission, mailing, or in-person delivery. Many institutions prefer electronic submissions due to their efficiency and ease of tracking. Ensure that you follow the specific submission guidelines provided by the regulatory authority or the institution overseeing the clinical trial. Each method may have different requirements, so it is important to verify the preferred approach before submission.
Quick guide on how to complete form 1572 2016 2019
Uncover the easiest method to complete and endorse your Form 1572
Are you still spending time assembling your official documents on paper instead of doing it online? airSlate SignNow provides a superior approach to complete and endorse your Form 1572 and comparable forms for public services. Our advanced eSignature solution equips you with everything necessary to manage documents swiftly and in compliance with official standards - comprehensive PDF editing, organizing, securing, signing, and sharing tools all available within a user-friendly interface.
Only a few steps are needed to finish filling out and signing your Form 1572:
- Insert the fillable template into the editor with the Get Form option.
- Verify what information you need to include in your Form 1572.
- Switch between the fields using the Next option to ensure nothing is overlooked.
- Employ Text, Check, and Cross tools to populate the fields with your data.
- Modify the content using Text boxes or Images from the upper toolbar.
- Emphasize what is essential or Remove sections that are no longer relevant.
- Click on Sign to create a legally binding eSignature using your preferred method.
- Insert the Date beside your signature and complete your task with the Done option.
Store your completed Form 1572 in the Documents folder of your profile, download it, or transfer it to your chosen cloud storage. Our solution also provides adaptable form sharing. There’s no need to print your forms when needing to submit them to the relevant public office - do it via email, fax, or by requesting a USPS “snail mail” delivery from your account. Try it out now!
Create this form in 5 minutes or less
FAQs
-
How do I fill out 2016 ITR form?
First of all you must know about all of your sources of income. In Indian Income Tax Act there are multiple forms for different types of sources of Income. If you have only salary & other source of income you can fill ITR-1 by registering your PAN on e-Filing Home Page, Income Tax Department, Government of India after registration you have to login & select option fill ITR online in this case you have to select ITR-1 for salary, house property & other source income.if you have income from business & profession and not maintaining books & also not mandatory to prepare books & total turnover in business less than 1 Crores & want to show profit more than 8% & if you are a professional and not required to make books want to show profit more than 50% of receipts than you can use online quick e-filling form ITR-4S i.s. for presumptive business income.for other source of income there are several forms according to source of income download Excel utility or JAVA utility form e-Filing Home Page, Income Tax Department, Government of India fill & upload after login to your account.Prerequisite before E-filling.Last year return copy (if available)Bank Account number with IFSC Code.Form 16/16A (if Available)Saving Details / Deduction Slips LIC,PPF, etc.Interest Statement from Banks or OthersProfit & Loss Account, Balance Sheet, Tax Audit Report only if filling ITR-4, ITR-5, ITR-6, ITR-7.hope this will help you in case any query please let me know.
-
How do I fill out a CLAT 2019 application form?
Hi thereFirst of all, let me tell you some important points:CLAT 2019 has gone OFFLINE this yearBut the application forms for CLAT 2019 have to be filled ONLINEThe payment of the application fees also should be made onlineNow, kindly note the important dates:Note the details regarding the application fees:Here, if you want the Previous Year Question papers, Rs.500 would be added to the application fees.Apart from this, there would be bank transaction charges added to the application fees.The application fees is non-refundable.Note one important point here that before you fill the application form, check for your eligibility.To know the complete details of filling the application form along with other information like the eligibility - in terms of age, educational qualification, list of NLUs participating and the seats offered under each category, CLAT Reservation policies, CLAT exam pattern, marking scheme, syllabus, important documents required to be kept ready before filling the form, kindly go to the link below:How to fill CLAT 2019 Application form? Registration OPEN | All you need to knowTo know how to prepare for one of the very important section of CLAT exam, GK and Current Affairs, kindly go to the link below:How to prepare GK & Current Affairs for CLAT 2019To practice, daily MCQs on Current Affairs, kindly go to the link below:#CLAT2019 | #AILET2019 | #DULLB2019 | GK and Current Affairs Series: 5 in 10 Series: Day 12For any other queries, kindly write to us at mailateasyway@gmail.comThanks & Regards!
-
How do I fill out the NEET 2019 application form?
Expecting application form of NEET2019 will be same as that of NEET2018, follow the instructions-For Feb 2019 Exam:EventsDates (Announced)Release of application form-1st October 2018Application submission last date-31st October 2018Last date to pay the fee-Last week of October 2018Correction Window Open-1st week of November 2018Admit card available-1st week of January 2019Exam date-3rd February to 17th February 2019Answer key & OMR release-Within a week after examAnnouncement of result-1st week of March 2019Counselling begins-2nd week of June 2019For May 2019 Exam:EventsDates (Announced)Application form Release-2nd week of March 2019Application submission last date-2nd week of April 2019Last date to pay the fee-2nd week of April 2019Correction Window Open-3rd week of April 2019Admit card available-1st week of May 2019Exam date-12th May to 26th May 2019Answer key & OMR release-Within a week after examAnnouncement of result-1st week of June 2019Counselling begins-2nd week of June 2019NEET 2019 Application FormCandidates should fill the application form as per the instructions given in the information bulletin. Below we are providing NEET 2019 application form details:The application form will be issued through online mode only.No application will be entertained through offline mode.NEET UG registration 2019 will be commenced from the 1st October 2018 (Feb Exam) & second week of March 2018 (May Exam).Candidates should upload the scanned images of recent passport size photograph and signature.After filling the application form completely, a confirmation page will be generated. Download it.There will be no need to send the printed confirmation page to the board.Application Fee:General and OBC candidates will have to pay Rs. 1400/- as an application fee.The application fee for SC/ST and PH candidates will be Rs. 750/-.Fee payment can be done through credit/debit card, net banking, UPI and e-wallet.Service tax will also be applicable.CategoryApplication FeeGeneral/OBC-1400/-SC/ST/PH-750/-Step 1: Fill the Application FormGo the official portal of the conducting authority (Link will be given above).Click on “Apply Online” link.A candidate has to read all the instruction and then click on “Proceed to Apply Online NEET (UG) 2019”.Step 1.1: New RegistrationFill the registration form carefully.Candidates have to fill their name, Mother’s Name, Father’s Name, Category, Date of Birth, Gender, Nationality, State of Eligibility (for 15% All India Quota), Mobile Number, Email ID, Aadhaar card number, etc.After filling all the details, two links will be given “Preview &Next” and “Reset”.If candidate satisfied with the filled information, then they have to click on “Next”.After clicking on Next Button, the information submitted by the candidate will be displayed on the screen. If information correct, click on “Next” button, otherwise go for “Back” button.Candidates may note down the registration number for further procedure.Now choose the strong password and re enter the password.Choose security question and feed answer.Enter the OTP would be sent to your mobile number.Submit the button.Step 1.2: Login & Application Form FillingLogin with your Registration Number and password.Fill personal details.Enter place of birth.Choose the medium of question paper.Choose examination centres.Fill permanent address.Fill correspondence address.Fill Details (qualification, occupation, annual income) of parents and guardians.Choose the option for dress code.Enter security pin & click on save & draft.Now click on preview and submit.Now, review your entries.Then. click on Final Submit.Step 2: Upload Photo and SignatureStep 2 for images upload will be appeared on screen.Now, click on link for Upload photo & signature.Upload the scanned images.Candidate should have scanned images of his latest Photograph (size of 10 Kb to 100 Kb.Signature(size of 3 Kb to 20 Kb) in JPEG format only.Step 3: Fee PaymentAfter uploading the images, candidate will automatically go to the link for fee payment.A candidate has to follow the instruction & submit the application fee.Choose the Bank for making payment.Go for Payment.Candidate can pay the fee through Debit/Credit Card/Net Banking/e-wallet (CSC).Step 4: Take the Printout of Confirmation PageAfter the fee payment, a candidate may take the printout of the confirmation page.Candidates may keep at least three copies of the confirmation page.Note:Must retain copy of the system generated Self Declaration in respect of candidates from J&K who have opted for seats under 15% All India Quota.IF any queries, feel free to comment..best of luck
-
How can I fill out the BITSAT Application Form 2019?
BITSAT 2019 Application Forms are available online. Students who are eligible for the admission test can apply online before 20 March 2018, 5 pm.Click here to apply for BITSAT 2019Step 1: Follow the link given aboveStep 2: Fill online application formPersonal Details12th Examination DetailsTest Centre PreferencesStep 3: Upload scanned photograph (4 kb to 50 kb) and signature ( 1 kb to 30 kb).Step 4: Pay application fee either through online payment mode or through e-challan (ICICI Bank)BITSAT-2019 Application FeeMale Candidates - Rs. 3150/-Female Candidates - Rs. 2650/-Thanks!
-
How can I fill out the COMEDK 2019 application form?
COMEDK 2019 application is fully online based and there is no need to send the application by post or by any other method. Check the below-mentioned guidelines to register for the COMEDK 2019 exam:Step 1 Visit the official website of the COMEDK UGET- comedk.orgStep 2 Click on “Engineering Application”.Step 3 After that click on “Login or Register” button.Step 4 You will be asked to enter the Application SEQ Number/User ID and Password. But since you have not registered. You need to click on the “Click here for Registration”.Step 5 Fill in the required details like “Full Name”, “DOB”, “Unique Photo ID Proof”, “Photo ID Proof Number”, “Email ID” and “Mobile Number”.Step 6 Then click on the “Generate OTP”Step 7 After that you need to enter the captcha code and then an OTP will be sent to the mobile number that you have provided.Step 8 A new window having your previously entered registration details will open where you need to enter the OTP.Step 9 Re-check all the details, enter the captcha code and click on the “Register” button.Step 10 After that a page will appear where you will be having the User ID and all the details that you entered. Also, you will be notified that you have successfully registered yourself and a User ID and Password will be sent to your mobile number and email ID.COMEDK 2019 Notification | Steps To Apply For COMEDK UGET ExamCheck the below-mentioned guidelines to fill COMEDK Application Form after COMEDK Login.Step 1 Using your User ID and Password. Log in using the User ID and passwordStep 2 You will be shown that your application form is incomplete. So you need to go to the topmost right corner and click on the “Go to application” tab.Step 3 Go to the COMEDK official website and login with these credentials.Step 4 After that click on “Go to application form”.Step 5 Select your preferred stream and course.Step 6 Click on “Save and Continue”.Step 7 Carefully enter your Personal, Category and Academic details.Step 8 Upload your Photograph and Signature, Parents Signature, your ID Proof, and Declaration.Step 9 Enter your “Payment Mode” and “Amount”.Step 10 Enter “Security code”.Step 11 Tick the “I Agree” checkbox.Step 12 Click on the “Submit” button.
-
How can I fill out the application form for the JMI (Jamia Millia Islamia) 2019?
Form for jamia school have been releaseYou can fill it from jamia siteJamia Millia Islamia And for collegeMost probably the form will out end of this month or next monthBut visit the jamia site regularly.Jamia Millia Islamiacheck whether the form is out or not for the course you want to apply.when notification is out then you have to create the account for entrance and for 2 entrance same account will be used you have to check in the account that the course you want to apply is there in listed or not ….if not then you have to create the different account for that course .If you have any doubts you can freely ask me .
Create this form in 5 minutes!
How to create an eSignature for the form 1572 2016 2019
How to create an electronic signature for the Form 1572 2016 2019 online
How to create an electronic signature for the Form 1572 2016 2019 in Google Chrome
How to make an eSignature for putting it on the Form 1572 2016 2019 in Gmail
How to make an eSignature for the Form 1572 2016 2019 from your smartphone
How to generate an electronic signature for the Form 1572 2016 2019 on iOS devices
How to generate an electronic signature for the Form 1572 2016 2019 on Android OS
People also ask
-
What is Form 1572 and why is it important?
Form 1572 is a key document used in clinical trials that outlines the necessary information about the study and investigators involved. It is crucial for compliance with regulations set forth by the FDA. Using airSlate SignNow, you can easily manage and eSign Form 1572 to ensure that all parties are in agreement and that the document meets regulatory requirements.
-
How can airSlate SignNow help with completing Form 1572?
airSlate SignNow simplifies the process of completing Form 1572 by allowing users to fill out, sign, and send the document electronically. Our platform ensures that the entire process is streamlined and compliant, reducing the risk of errors. With templates and easy access to necessary data, you can save time and enhance efficiency when working with Form 1572.
-
What are the pricing options for using airSlate SignNow for Form 1572?
airSlate SignNow offers flexible pricing plans that cater to different business needs, making it cost-effective to manage Form 1572 and other documents. You can choose from various subscription models depending on your organization’s size and volume of document transactions. This ensures that you only pay for what you need while gaining access to powerful features.
-
Can I integrate airSlate SignNow with other software for Form 1572 management?
Yes, airSlate SignNow seamlessly integrates with various software tools, enhancing your workflow for managing Form 1572. Whether you use CRM systems, project management tools, or cloud storage services, our integrations ensure you can access and send your documents effortlessly. This interconnected approach helps streamline the entire document management process.
-
What features does airSlate SignNow offer for managing Form 1572?
airSlate SignNow provides a range of features specifically designed to facilitate the management of Form 1572. These include customizable templates, real-time tracking, and secure electronic signatures. Additionally, our platform ensures compliance with legal standards, making it easier to manage your documents with confidence.
-
Is airSlate SignNow secure for handling sensitive information in Form 1572?
Absolutely. airSlate SignNow prioritizes security and complies with industry standards to protect sensitive information in Form 1572. Our platform utilizes advanced encryption methods and secure storage solutions, ensuring that your documents remain safe from unauthorized access throughout the signing process.
-
Can I use airSlate SignNow on mobile devices for Form 1572?
Yes, airSlate SignNow is fully optimized for mobile devices, allowing you to manage Form 1572 on-the-go. Whether you are using a smartphone or tablet, you can easily fill out, sign, and send your documents from anywhere. This flexibility ensures that your workflow remains uninterrupted and efficient.
Get more for Form 1572
- Criminal record check forms
- Modulo denuncia furto form
- Rt edgar online application form
- North greenville university transcript request form
- Personal representatives deed of distribution colorado probate form
- Hospice recertification note example form
- Romero motion template form
- Antenuptial agreement template 787739044 form
Find out other Form 1572
- Electronic signature Texas Police Lease Termination Letter Safe
- How To Electronic signature Texas Police Stock Certificate
- How Can I Electronic signature Wyoming Real Estate Quitclaim Deed
- Electronic signature Virginia Police Quitclaim Deed Secure
- How Can I Electronic signature West Virginia Police Letter Of Intent
- How Do I Electronic signature Washington Police Promissory Note Template
- Electronic signature Wisconsin Police Permission Slip Free
- Electronic signature Minnesota Sports Limited Power Of Attorney Fast
- Electronic signature Alabama Courts Quitclaim Deed Safe
- How To Electronic signature Alabama Courts Stock Certificate
- Can I Electronic signature Arkansas Courts Operating Agreement
- How Do I Electronic signature Georgia Courts Agreement
- Electronic signature Georgia Courts Rental Application Fast
- How Can I Electronic signature Hawaii Courts Purchase Order Template
- How To Electronic signature Indiana Courts Cease And Desist Letter
- How Can I Electronic signature New Jersey Sports Purchase Order Template
- How Can I Electronic signature Louisiana Courts LLC Operating Agreement
- How To Electronic signature Massachusetts Courts Stock Certificate
- Electronic signature Mississippi Courts Promissory Note Template Online
- Electronic signature Montana Courts Promissory Note Template Now