PVDOMICS STUDY Annual Follow Up Form #187 2023-2026


What is the PVDOMICS STUDY Annual Follow up Form #187
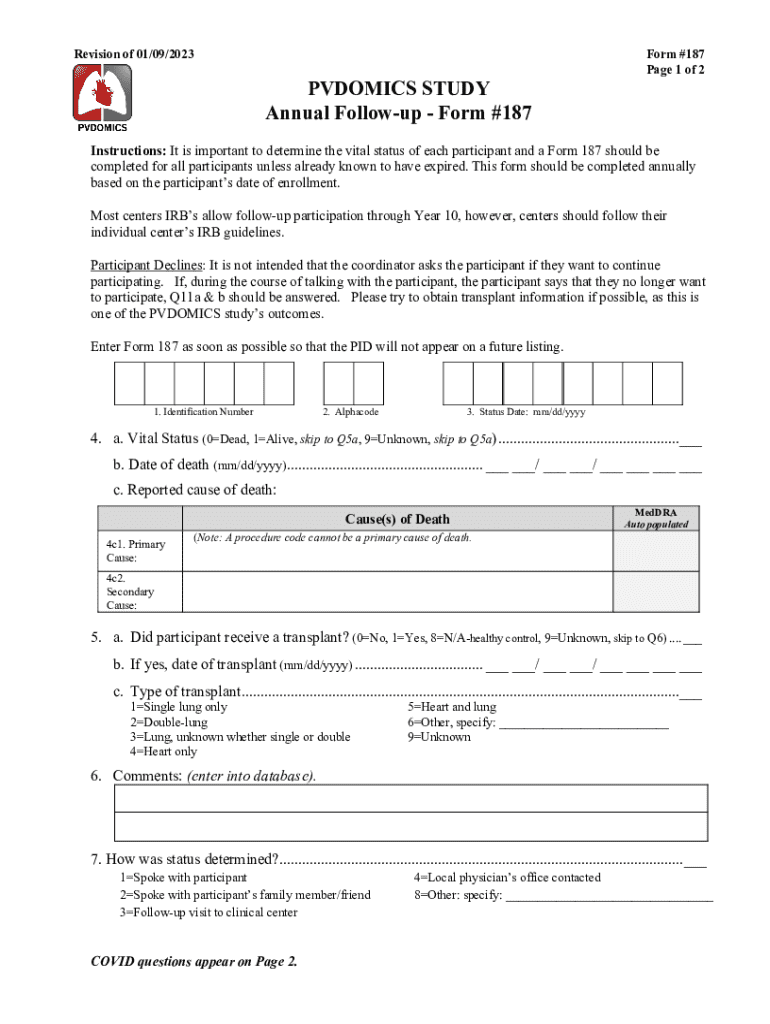
The PVDOMICS STUDY Annual Follow up Form #187 is a specialized document designed for participants in the PVDOMICS study, which focuses on understanding and improving outcomes for individuals with peripheral vascular disease. This form collects vital information regarding the participant's health status, treatment adherence, and overall well-being since their last assessment. It plays a crucial role in tracking the long-term effects of various interventions and ensuring that participants receive appropriate care and support.
How to use the PVDOMICS STUDY Annual Follow up Form #187
To effectively use the PVDOMICS STUDY Annual Follow up Form #187, participants should first ensure they have the most recent version of the form. The form typically requires personal information, health history updates, and details about any new treatments or medications. Participants should read each section carefully and provide accurate information to facilitate proper analysis. Once completed, the form can be submitted electronically or by mail, depending on the guidelines provided by the study coordinators.
Steps to complete the PVDOMICS STUDY Annual Follow up Form #187
Completing the PVDOMICS STUDY Annual Follow up Form #187 involves several straightforward steps:
- Gather necessary personal information, including your contact details and medical history.
- Review any previous forms or assessments to ensure consistency in your responses.
- Fill out each section of the form, providing detailed and accurate information.
- Double-check your entries for any errors or omissions.
- Submit the completed form as instructed, either electronically or via mail.
Key elements of the PVDOMICS STUDY Annual Follow up Form #187
The key elements of the PVDOMICS STUDY Annual Follow up Form #187 include:
- Participant identification information, such as name and study ID.
- Health status updates, including any changes in symptoms or conditions.
- Details about treatments received since the last follow-up.
- Information on lifestyle factors, such as diet and exercise habits.
- Any new medications or therapies initiated during the year.
Legal use of the PVDOMICS STUDY Annual Follow up Form #187
The PVDOMICS STUDY Annual Follow up Form #187 is governed by specific legal and ethical guidelines to protect participant confidentiality and ensure data integrity. Participants must consent to the use of their information for research purposes, and the form must comply with applicable regulations, including the Health Insurance Portability and Accountability Act (HIPAA). Proper handling and storage of the completed forms are essential to maintain participant privacy.
Form Submission Methods
Participants can submit the PVDOMICS STUDY Annual Follow up Form #187 through various methods, depending on the instructions provided by the study coordinators. Common submission methods include:
- Online submission through a secure portal.
- Mailing the completed form to the designated address.
- In-person submission at specified study locations or events.
Quick guide on how to complete pvdomics study annual follow up form 187
Complete PVDOMICS STUDY Annual Follow up Form #187 effortlessly on any device
Online document management has gained popularity among businesses and individuals. It offers an ideal environmentally friendly alternative to traditional printed and signed forms, allowing you to easily locate the correct template and securely store it online. airSlate SignNow equips you with all the necessary tools to create, modify, and electronically sign your documents quickly and efficiently. Handle PVDOMICS STUDY Annual Follow up Form #187 on any device using airSlate SignNow's Android or iOS applications and streamline your document-related tasks today.
The simplest way to modify and eSign PVDOMICS STUDY Annual Follow up Form #187 with ease
- Locate PVDOMICS STUDY Annual Follow up Form #187 and click Get Form to begin.
- Utilize the tools we provide to complete your form.
- Emphasize important sections of the documents or redact sensitive information using specialized tools that airSlate SignNow offers for this purpose.
- Create your eSignature with the Sign feature, which takes mere seconds and carries the same legal validity as a conventional wet ink signature.
- Review the information and click the Done button to save your modifications.
- Choose how you wish to submit your form, via email, SMS, or invitation link, or download it to your computer.
Say goodbye to lost or misplaced documents, tedious form searching, or mistakes that necessitate printing new document copies. airSlate SignNow meets all your document management needs in just a few clicks from your preferred device. Alter and eSign PVDOMICS STUDY Annual Follow up Form #187 and ensure excellent communication at every step of your form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Find and fill out the correct pvdomics study annual follow up form 187
Create this form in 5 minutes!
How to create an eSignature for the pvdomics study annual follow up form 187
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is the PVDOMICS STUDY Annual Follow up Form #187?
The PVDOMICS STUDY Annual Follow up Form #187 is a specialized document designed to collect essential data for the PVDOMICS study. It streamlines the process of gathering patient information, ensuring accuracy and compliance. By utilizing this form, researchers can efficiently track patient progress over time.
-
How can I access the PVDOMICS STUDY Annual Follow up Form #187?
You can easily access the PVDOMICS STUDY Annual Follow up Form #187 through the airSlate SignNow platform. Simply sign up for an account, and you will have immediate access to this form along with other essential documents. Our user-friendly interface makes it simple to find and utilize the form.
-
What are the benefits of using the PVDOMICS STUDY Annual Follow up Form #187?
Using the PVDOMICS STUDY Annual Follow up Form #187 offers numerous benefits, including improved data collection and enhanced patient tracking. It helps researchers maintain organized records and ensures that all necessary information is captured efficiently. This ultimately contributes to the success of the PVDOMICS study.
-
Is the PVDOMICS STUDY Annual Follow up Form #187 customizable?
Yes, the PVDOMICS STUDY Annual Follow up Form #187 can be customized to meet specific research needs. airSlate SignNow allows users to modify fields and add additional questions as necessary. This flexibility ensures that the form aligns perfectly with your study requirements.
-
What integrations are available for the PVDOMICS STUDY Annual Follow up Form #187?
The PVDOMICS STUDY Annual Follow up Form #187 integrates seamlessly with various applications, enhancing its functionality. You can connect it with CRM systems, cloud storage, and other tools to streamline your workflow. This integration capability makes data management more efficient.
-
How much does it cost to use the PVDOMICS STUDY Annual Follow up Form #187?
The cost of using the PVDOMICS STUDY Annual Follow up Form #187 varies based on your subscription plan with airSlate SignNow. We offer competitive pricing that provides excellent value for the features included. For detailed pricing information, please visit our website or contact our sales team.
-
Can I track responses from the PVDOMICS STUDY Annual Follow up Form #187?
Absolutely! airSlate SignNow provides robust tracking features for the PVDOMICS STUDY Annual Follow up Form #187. You can monitor responses in real-time, ensuring that you stay updated on patient data and can act promptly on any necessary follow-ups.
Get more for PVDOMICS STUDY Annual Follow up Form #187
- Emp5627 form
- Wsu status check form
- Icu university online application form
- The boy in the striped pajamas chapter questions and answers pdf form
- Model imputernicire banca transilvania form
- Class 3 english worksheet pdf 389344578 form
- T dependents request for change of program or place of training under provisions of chapters 33 and 35 title 38 u s c form
- Grant funding agreement template form
Find out other PVDOMICS STUDY Annual Follow up Form #187
- Can I eSign Washington Charity LLC Operating Agreement
- eSign Wyoming Charity Living Will Simple
- eSign Florida Construction Memorandum Of Understanding Easy
- eSign Arkansas Doctors LLC Operating Agreement Free
- eSign Hawaii Construction Lease Agreement Mobile
- Help Me With eSign Hawaii Construction LLC Operating Agreement
- eSign Hawaii Construction Work Order Myself
- eSign Delaware Doctors Quitclaim Deed Free
- eSign Colorado Doctors Operating Agreement Computer
- Help Me With eSign Florida Doctors Lease Termination Letter
- eSign Florida Doctors Lease Termination Letter Myself
- eSign Hawaii Doctors Claim Later
- eSign Idaho Construction Arbitration Agreement Easy
- eSign Iowa Construction Quitclaim Deed Now
- How Do I eSign Iowa Construction Quitclaim Deed
- eSign Louisiana Doctors Letter Of Intent Fast
- eSign Maine Doctors Promissory Note Template Easy
- eSign Kentucky Construction Claim Online
- How Can I eSign Maine Construction Quitclaim Deed
- eSign Colorado Education Promissory Note Template Easy