AVOXimeter 1000E Quality Control Record Sheet Note Form


What is the AVOXimeter 1000E Quality Control Record Sheet Note
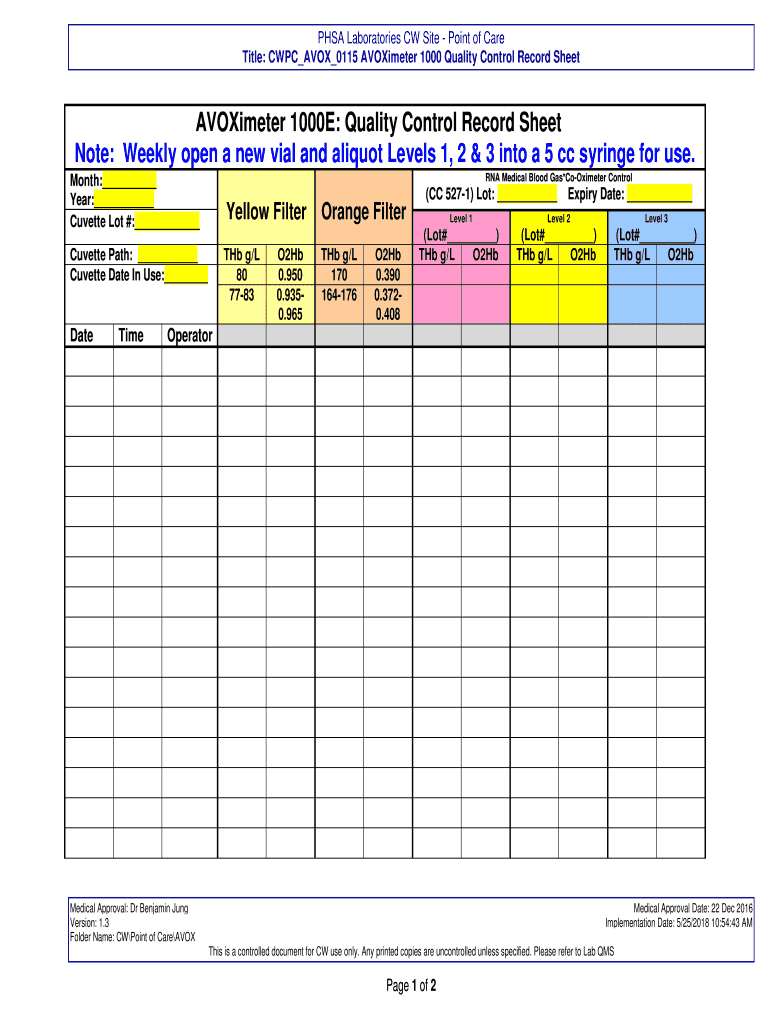
The AVOXimeter 1000E Quality Control Record Sheet Note is a critical document used in the healthcare sector to ensure the accuracy and reliability of blood gas analysis. This record sheet is specifically designed for use with the AVOXimeter 1000E device, which measures various parameters, including oxygen saturation and carbon dioxide levels in the blood. The quality control record sheet helps healthcare professionals document the calibration and performance checks performed on the device, ensuring compliance with industry standards and regulations.
How to use the AVOXimeter 1000E Quality Control Record Sheet Note
Using the AVOXimeter 1000E Quality Control Record Sheet Note involves several straightforward steps. First, healthcare professionals should obtain the record sheet, which can be printed or accessed digitally. Next, they should fill in essential information such as the date, time, and operator's name. During the quality control process, operators must conduct necessary tests and record the results on the sheet. This documentation serves as a reference for future audits and ensures that the device is functioning correctly. Regularly reviewing these records can help identify trends or issues that may require attention.
Key elements of the AVOXimeter 1000E Quality Control Record Sheet Note
Several key elements are essential for effective documentation on the AVOXimeter 1000E Quality Control Record Sheet Note. These include:
- Date and time: Documenting when the quality control checks were performed.
- Operator identification: Recording the name or initials of the person conducting the tests.
- Test results: Clearly noting the outcomes of each quality control test conducted.
- Calibration status: Indicating whether the device was calibrated correctly before testing.
- Comments: Providing any additional notes that may be relevant to the quality control process.
Steps to complete the AVOXimeter 1000E Quality Control Record Sheet Note
Completing the AVOXimeter 1000E Quality Control Record Sheet Note involves a series of methodical steps:
- Gather the necessary materials, including the record sheet and the AVOXimeter 1000E device.
- Perform the quality control tests as per the manufacturer's guidelines.
- Document the results on the record sheet, ensuring accuracy and clarity.
- Review the completed record for any discrepancies or missing information.
- Store the record sheet securely for future reference and compliance checks.
Legal use of the AVOXimeter 1000E Quality Control Record Sheet Note
The AVOXimeter 1000E Quality Control Record Sheet Note is vital for legal compliance in healthcare settings. Accurate and thorough documentation is essential to meet regulatory standards set by bodies such as the Food and Drug Administration (FDA) and the Clinical Laboratory Improvement Amendments (CLIA). Maintaining these records not only supports operational integrity but also protects healthcare providers in case of audits or legal inquiries. Failure to keep proper records may result in penalties or loss of accreditation.
Examples of using the AVOXimeter 1000E Quality Control Record Sheet Note
Examples of using the AVOXimeter 1000E Quality Control Record Sheet Note can be found in various healthcare settings. For instance, a hospital laboratory may use the record sheet daily to document quality control checks on the AVOXimeter 1000E before performing patient tests. In outpatient clinics, healthcare professionals might use the sheet weekly to ensure the device is functioning correctly. These records provide a clear history of the device's performance and can be invaluable during inspections or quality assurance reviews.
Quick guide on how to complete avoximeter 1000e quality control record sheet note
Complete AVOXimeter 1000E Quality Control Record Sheet Note effortlessly on any device
Online document management has become increasingly popular with companies and individuals alike. It offers an ideal environmentally-friendly substitute for conventional printed and signed documents, as you can easily locate the necessary form and safely store it online. airSlate SignNow provides all the resources you need to create, modify, and eSign your documents quickly and efficiently. Manage AVOXimeter 1000E Quality Control Record Sheet Note on any platform using airSlate SignNow's Android or iOS applications and simplify your document-based tasks today.
How to alter and eSign AVOXimeter 1000E Quality Control Record Sheet Note with ease
- Locate AVOXimeter 1000E Quality Control Record Sheet Note and click on Get Form to begin.
- Utilize the tools we provide to complete your form.
- Emphasize relevant parts of your documents or redact sensitive information with tools that airSlate SignNow specifically offers for this purpose.
- Generate your signature using the Sign tool, which takes mere seconds and carries the same legal validity as a conventional wet ink signature.
- Review the details and click on the Done button to save your modifications.
- Choose how you want to send your form, via email, text message (SMS), or invite link, or download it to your PC.
Eliminate concerns about lost or misplaced documents, tedious form searches, or errors that necessitate the printing of new copies. airSlate SignNow meets all your document management needs in just a few clicks from any device you prefer. Modify and eSign AVOXimeter 1000E Quality Control Record Sheet Note and ensure seamless communication at every stage of your form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the avoximeter 1000e quality control record sheet note
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is the AVOXimeter 1000E Quality Control Record Sheet Note?
The AVOXimeter 1000E Quality Control Record Sheet Note is a comprehensive document designed to ensure accurate and reliable quality control for AVOXimeter 1000E devices. It helps users maintain compliance with industry standards and provides a clear record of quality checks performed on the device.
-
How can I purchase the AVOXimeter 1000E Quality Control Record Sheet Note?
You can purchase the AVOXimeter 1000E Quality Control Record Sheet Note directly through our website or authorized distributors. Pricing may vary based on the quantity and any applicable discounts, so be sure to check for the best offers available.
-
What features does the AVOXimeter 1000E Quality Control Record Sheet Note include?
The AVOXimeter 1000E Quality Control Record Sheet Note includes sections for recording calibration data, maintenance logs, and performance checks. It is designed to be user-friendly, ensuring that all necessary information is captured efficiently and accurately.
-
What are the benefits of using the AVOXimeter 1000E Quality Control Record Sheet Note?
Using the AVOXimeter 1000E Quality Control Record Sheet Note enhances the reliability of your quality control processes. It helps in tracking device performance over time, ensuring compliance with regulatory requirements, and ultimately improving patient safety.
-
Is the AVOXimeter 1000E Quality Control Record Sheet Note compatible with other systems?
Yes, the AVOXimeter 1000E Quality Control Record Sheet Note can be integrated with various electronic health record (EHR) systems and quality management software. This compatibility allows for seamless data transfer and improved workflow efficiency.
-
How often should I use the AVOXimeter 1000E Quality Control Record Sheet Note?
It is recommended to use the AVOXimeter 1000E Quality Control Record Sheet Note regularly, ideally with each calibration and maintenance session. This ensures that all quality control measures are documented and any issues are addressed promptly.
-
Can I customize the AVOXimeter 1000E Quality Control Record Sheet Note for my facility?
Absolutely! The AVOXimeter 1000E Quality Control Record Sheet Note can be customized to meet the specific needs of your facility. You can add additional fields or modify existing ones to better align with your quality control protocols.
Get more for AVOXimeter 1000E Quality Control Record Sheet Note
- Connecticut a corporation form
- Connecticut sample certificate form
- Connecticut llc 497301369 form
- Pllc notices and resolutions connecticut form
- Sample transmittal letter template form
- New resident guide connecticut form
- Satisfaction release or cancellation of mortgage by corporation connecticut form
- Satisfaction release or cancellation of mortgage by individual connecticut form
Find out other AVOXimeter 1000E Quality Control Record Sheet Note
- eSign Pennsylvania Property management lease agreement Secure
- eSign Hawaii Rental agreement for house Fast
- Help Me With eSign Virginia Rental agreement contract
- eSign Alaska Rental lease agreement Now
- How To eSign Colorado Rental lease agreement
- How Can I eSign Colorado Rental lease agreement
- Can I eSign Connecticut Rental lease agreement
- eSign New Hampshire Rental lease agreement Later
- Can I eSign North Carolina Rental lease agreement
- How Do I eSign Pennsylvania Rental lease agreement
- How To eSign South Carolina Rental lease agreement
- eSign Texas Rental lease agreement Mobile
- eSign Utah Rental agreement lease Easy
- How Can I eSign North Dakota Rental lease agreement forms
- eSign Rhode Island Rental lease agreement forms Now
- eSign Georgia Rental lease agreement template Simple
- Can I eSign Wyoming Rental lease agreement forms
- eSign New Hampshire Rental lease agreement template Online
- eSign Utah Rental lease contract Free
- eSign Tennessee Rental lease agreement template Online