Attachment E CDRH Final Guidance Cover Sheet FDA Form


What is the CDRH cover sheet?
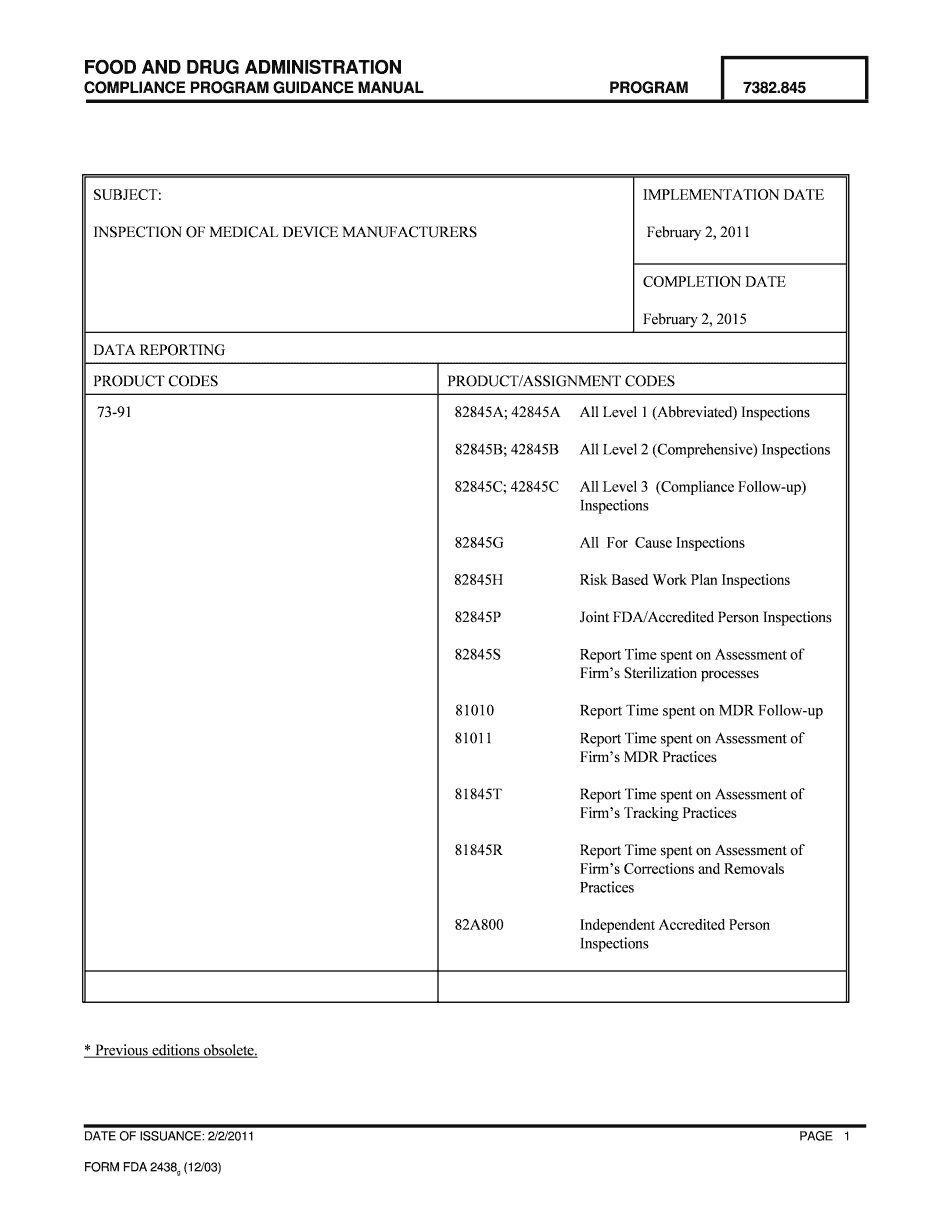
The CDRH cover sheet is a crucial document used in the submission process for medical devices and related products to the FDA. It serves as a summary of the submission, providing essential information about the applicant, the product, and the intended use. The cover sheet is part of the Attachment E to the CDRH Final Guidance, which outlines the requirements for submissions to ensure compliance with FDA regulations. This document is vital for facilitating the review process by clearly presenting key details to FDA reviewers.
Steps to complete the CDRH cover sheet
Completing the CDRH cover sheet involves several important steps to ensure accuracy and compliance. Begin by gathering all necessary information about your product and submission. Follow these steps:
- Provide the applicant's name and contact information.
- Include the product's name, common name, and classification.
- Specify the type of submission, such as a premarket notification (510(k)) or premarket approval (PMA).
- Detail the intended use and indications for the device.
- List any relevant prior submissions or related documents.
Review the completed cover sheet for accuracy before submission, as errors can lead to delays in the review process.
Key elements of the CDRH cover sheet
The CDRH cover sheet contains several key elements that are essential for a successful submission. These include:
- Applicant Information: Name, address, and contact details of the submitting party.
- Device Identification: Product name, common name, and classification information.
- Submission Type: Indication of whether the submission is a 510(k), PMA, or other type.
- Intended Use: Clear description of the device's purpose and indications.
- Regulatory History: Information on any previous submissions related to the device.
Including these elements accurately helps ensure that the FDA can efficiently process the submission.
Legal use of the CDRH cover sheet
The CDRH cover sheet is legally binding when submitted as part of an official application to the FDA. It must comply with the relevant regulations set forth by the FDA to ensure that all information provided is truthful and accurate. Misrepresentation or failure to disclose necessary information can lead to penalties, including rejection of the submission or legal repercussions. Therefore, it is essential to understand the legal implications of the information presented on the cover sheet.
Form submission methods for the CDRH cover sheet
The CDRH cover sheet can be submitted through various methods, depending on the type of submission. Common submission methods include:
- Online Submission: Many submissions can be made electronically through the FDA's electronic submission gateway.
- Mail: Physical copies of the cover sheet and accompanying documents can be sent via postal service.
- In-Person: Some applicants may choose to deliver their submissions directly to the FDA office.
Each method has specific requirements and timelines, so it is important to choose the most appropriate option for your submission.
Examples of using the CDRH cover sheet
Examples of using the CDRH cover sheet can provide clarity on its application. For instance, a manufacturer submitting a new medical device for approval would fill out the cover sheet with details about the device, including its classification and intended use. Another example includes a company seeking to modify an existing device, where the cover sheet would reflect the changes and provide context for the FDA review. These examples illustrate how the cover sheet is tailored to the specific submission type and helps facilitate the regulatory process.
Quick guide on how to complete attachment e cdrh final guidance cover sheet fda
Complete Attachment E CDRH Final Guidance Cover Sheet FDA effortlessly on any device
Online document management has gained popularity among organizations and individuals alike. It offers an ideal eco-friendly alternative to traditional printed and signed forms, allowing you to access the necessary documents and securely save them online. airSlate SignNow provides you with all the resources required to create, modify, and electronically sign your documents quickly without delays. Manage Attachment E CDRH Final Guidance Cover Sheet FDA on any device using airSlate SignNow's Android or iOS applications and enhance your document-related processes today.
How to modify and electronically sign Attachment E CDRH Final Guidance Cover Sheet FDA with ease
- Find Attachment E CDRH Final Guidance Cover Sheet FDA and then click Get Form to begin.
- Utilize the tools we offer to complete your document.
- Emphasize important sections of your documents or redact sensitive data with tools provided by airSlate SignNow specifically for these tasks.
- Generate your electronic signature using the Sign tool, which takes only seconds and holds the same legal validity as a conventional handwritten signature.
- Review the details and then click on the Done button to save your changes.
- Choose how you would like to send your form, whether by email, SMS, or invitation link, or download it to your computer.
Say goodbye to lost or misplaced documents, tiring searches for forms, or mistakes that require new document copies. airSlate SignNow fulfills all your document management needs in just a few clicks from any device you prefer. Modify and electronically sign Attachment E CDRH Final Guidance Cover Sheet FDA and ensure excellent communication at every stage of your form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the attachment e cdrh final guidance cover sheet fda
How to make an electronic signature for the Attachment E Cdrh Final Guidance Cover Sheet Fda in the online mode
How to make an eSignature for the Attachment E Cdrh Final Guidance Cover Sheet Fda in Chrome
How to create an eSignature for signing the Attachment E Cdrh Final Guidance Cover Sheet Fda in Gmail
How to generate an eSignature for the Attachment E Cdrh Final Guidance Cover Sheet Fda straight from your smart phone
How to create an eSignature for the Attachment E Cdrh Final Guidance Cover Sheet Fda on iOS devices
How to create an electronic signature for the Attachment E Cdrh Final Guidance Cover Sheet Fda on Android devices
People also ask
-
What is a CDRH cover sheet?
A CDRH cover sheet is a document required by the FDA for submissions related to medical devices. It includes essential information about the submission, such as the type of device and contact details. Using airSlate SignNow, you can easily prepare, send, and electronically sign your CDRH cover sheet efficiently.
-
How can I create a CDRH cover sheet using airSlate SignNow?
Creating a CDRH cover sheet with airSlate SignNow is simple. Our platform provides customizable templates, allowing users to fill in required information and add electronic signatures. This streamlines the submission process, ensuring your documents are compliant and ready for review by the FDA.
-
Is there a cost associated with using airSlate SignNow for CDRH cover sheets?
Yes, airSlate SignNow offers flexible pricing plans tailored to different business needs. You'll find that our pricing structure is cost-effective, especially when preparing essential documents like CDRH cover sheets. We also provide a free trial to help you assess the platform before committing.
-
What features does airSlate SignNow offer for handling CDRH cover sheets?
airSlate SignNow includes robust features such as eSignature capabilities, document tracking, and secure cloud storage. These features make it easier for you to manage your CDRH cover sheets while ensuring compliance. Additionally, templates and automation help speed up your workflows.
-
Can airSlate SignNow integrate with other software for CDRH cover sheets?
Absolutely! airSlate SignNow integrates seamlessly with various applications like Google Drive, Dropbox, and CRM systems, enhancing your document management process. This integration allows you to pull data directly into your CDRH cover sheet, saving time and preventing errors.
-
What are the benefits of using airSlate SignNow for CDRH cover sheets?
Using airSlate SignNow for your CDRH cover sheets offers numerous benefits, including efficiency and compliance. The ability to eSign documents reduces turnaround time signNowly, while templates ensure you meet FDA requirements. Additionally, the user-friendly interface allows for a smooth experience.
-
How secure is the CDRH cover sheet data in airSlate SignNow?
Data security is a top priority at airSlate SignNow. Our platform employs industry-standard encryption and security protocols to protect your CDRH cover sheet and sensitive information. You can rest assured that your documents are safe and compliant with regulatory standards.
Get more for Attachment E CDRH Final Guidance Cover Sheet FDA
- Application for manufactured home community state of michigan michigan form
- Open public records act opra form
- Short answer questions chapter 1 form
- Structure contract template form
- Stucco contract template form
- Stud dog breed contract template form
- Stud dog contract template form
- Stud fee contract template form
Find out other Attachment E CDRH Final Guidance Cover Sheet FDA
- Electronic signature Kentucky Non-Profit Stock Certificate Online
- Electronic signature Legal PDF Louisiana Online
- Electronic signature Maine Legal Agreement Online
- Electronic signature Maine Legal Quitclaim Deed Online
- Electronic signature Missouri Non-Profit Affidavit Of Heirship Online
- Electronic signature New Jersey Non-Profit Business Plan Template Online
- Electronic signature Massachusetts Legal Resignation Letter Now
- Electronic signature Massachusetts Legal Quitclaim Deed Easy
- Electronic signature Minnesota Legal LLC Operating Agreement Free
- Electronic signature Minnesota Legal LLC Operating Agreement Secure
- Electronic signature Louisiana Life Sciences LLC Operating Agreement Now
- Electronic signature Oregon Non-Profit POA Free
- Electronic signature South Dakota Non-Profit Business Plan Template Now
- Electronic signature South Dakota Non-Profit Lease Agreement Template Online
- Electronic signature Legal Document Missouri Online
- Electronic signature Missouri Legal Claim Online
- Can I Electronic signature Texas Non-Profit Permission Slip
- Electronic signature Missouri Legal Rental Lease Agreement Simple
- Electronic signature Utah Non-Profit Cease And Desist Letter Fast
- Electronic signature Missouri Legal Lease Agreement Template Free