Serious Adverse Event Report Form SAE Form 2020-2026


Understanding the Serious Adverse Event Report Form
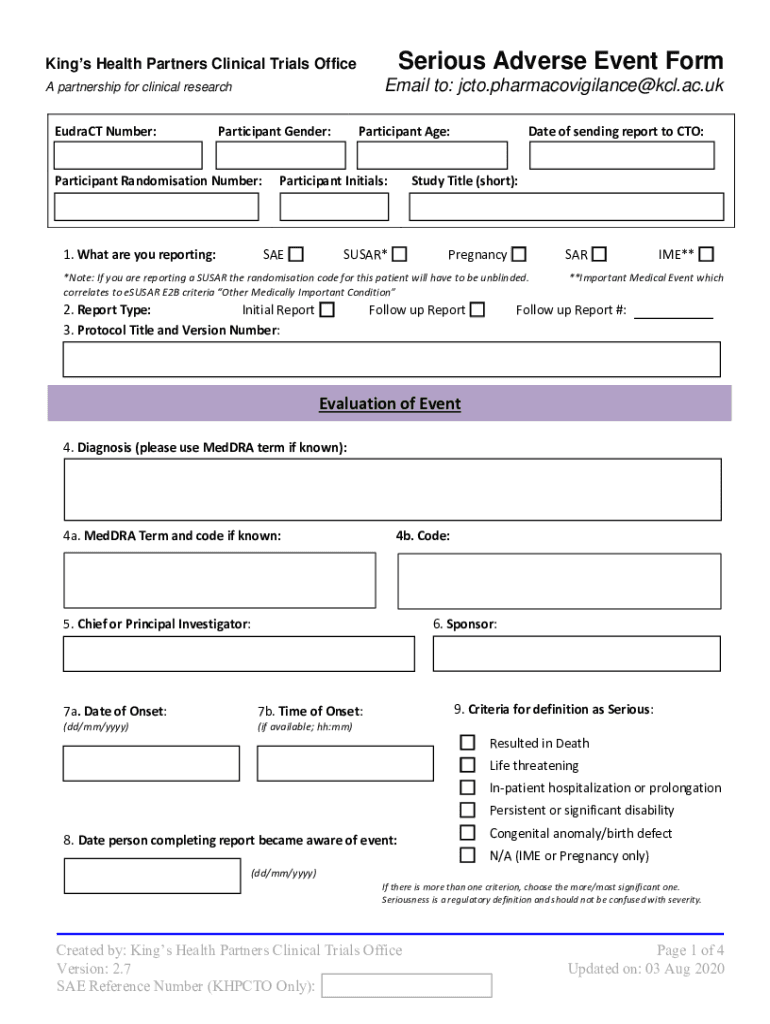
The Serious Adverse Event Report Form, often referred to as the SAE Form, is a crucial document used in clinical research to report any serious adverse events that occur during a study. This form is essential for maintaining participant safety and ensuring compliance with regulatory requirements. It captures detailed information about the event, including the nature of the event, the date it occurred, and any relevant medical history. Proper completion of this form is vital for the integrity of clinical trials and for safeguarding the welfare of participants.
Steps to Complete the Serious Adverse Event Report Form
Completing the Serious Adverse Event Report Form involves several key steps to ensure all necessary information is accurately captured. First, gather all relevant details about the adverse event, including the participant's identification information, the date of the event, and a description of the event itself. Next, document any medical history that may be pertinent to understanding the event. After filling in the required fields, review the form for accuracy and completeness before submission. It is important to follow any specific guidelines provided by the overseeing regulatory body or organization.
Key Elements of the Serious Adverse Event Report Form
The Serious Adverse Event Report Form includes several key elements that must be addressed to ensure comprehensive reporting. These elements typically include:
- Participant Information: Identification details of the participant involved in the adverse event.
- Event Description: A detailed account of the adverse event, including symptoms and severity.
- Timeline: Dates of occurrence and any relevant medical interventions.
- Medical History: Previous health conditions or treatments that may relate to the event.
- Outcome: The result of the adverse event, including any ongoing effects.
Legal Use of the Serious Adverse Event Report Form
The Serious Adverse Event Report Form is not only a tool for clinical researchers but also a legal document that must be handled with care. Proper use of this form ensures compliance with federal regulations, such as those set forth by the Food and Drug Administration (FDA) and the Office for Human Research Protections (OHRP). Failing to report adverse events can lead to significant legal repercussions, including penalties for non-compliance. Therefore, it is essential for researchers and institutions to adhere to the legal requirements surrounding the use of this form.
Examples of Using the Serious Adverse Event Report Form
Understanding how to effectively use the Serious Adverse Event Report Form can be enhanced by reviewing examples. For instance, if a participant experiences a severe allergic reaction during a clinical trial, the form would need to document the specifics of the reaction, the treatment provided, and any follow-up actions taken. Another example could involve a participant developing a serious health condition unrelated to the study, which must also be reported to ensure transparency and participant safety. These examples highlight the importance of thorough documentation in maintaining the integrity of clinical research.
Form Submission Methods
The Serious Adverse Event Report Form can typically be submitted through various methods, depending on the guidelines set by the overseeing institution or regulatory body. Common submission methods include:
- Online Submission: Many institutions provide a secure online portal for submitting the form.
- Mail: The form can be printed and sent via postal service to the appropriate regulatory office.
- In-Person Submission: Researchers may also have the option to deliver the form directly to the relevant office.
Quick guide on how to complete serious adverse event report form sae form
Complete Serious Adverse Event Report Form SAE Form seamlessly on any device
Digital document management has become increasingly popular among businesses and individuals. It offers an ideal eco-friendly substitute for conventional printed and signed papers, allowing you to obtain the necessary form and securely store it online. airSlate SignNow equips you with all the necessary tools to create, edit, and eSign your documents promptly without any delays. Manage Serious Adverse Event Report Form SAE Form on any platform using airSlate SignNow's Android or iOS applications and enhance any document-driven process today.
How to adjust and eSign Serious Adverse Event Report Form SAE Form effortlessly
- Obtain Serious Adverse Event Report Form SAE Form and then click Get Form to begin.
- Use the tools we provide to fill out your form.
- Emphasize important sections of your documents or redact sensitive information using tools that airSlate SignNow offers specifically for this purpose.
- Create your eSignature with the Sign tool, which takes only seconds and carries the same legal validity as a conventional wet ink signature.
- Review the details and then click on the Done button to save your changes.
- Choose how you would like to share your form, whether by email, text message (SMS), invitation link, or download it to your computer.
Eliminate concerns over lost or misplaced files, cumbersome form searches, or errors that necessitate printing new document copies. airSlate SignNow fulfills all your document management needs in just a few clicks from any device you prefer. Modify and eSign Serious Adverse Event Report Form SAE Form to ensure excellent communication at any stage of your document preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Find and fill out the correct serious adverse event report form sae form
Create this form in 5 minutes!
How to create an eSignature for the serious adverse event report form sae form
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is an adverse event form template?
An adverse event form template is a standardized document used to report any negative occurrences related to a product or service. This template helps organizations systematically collect and analyze data on adverse events, ensuring compliance and improving safety protocols.
-
How can airSlate SignNow help with adverse event form templates?
airSlate SignNow provides an easy-to-use platform for creating, sending, and eSigning adverse event form templates. With customizable features, you can tailor the template to meet your specific needs, ensuring that all necessary information is captured efficiently.
-
Is there a cost associated with using the adverse event form template on airSlate SignNow?
Yes, airSlate SignNow offers various pricing plans that include access to the adverse event form template. The cost is competitive and designed to provide businesses with a cost-effective solution for managing their documentation needs.
-
What features are included in the adverse event form template?
The adverse event form template includes features such as customizable fields, electronic signatures, and secure storage. These features streamline the reporting process and enhance data accuracy, making it easier for organizations to manage adverse events.
-
Can I integrate the adverse event form template with other software?
Absolutely! airSlate SignNow allows for seamless integration with various software applications, enabling you to connect your adverse event form template with your existing systems. This integration enhances workflow efficiency and data management.
-
What are the benefits of using an adverse event form template?
Using an adverse event form template simplifies the reporting process, ensures compliance, and improves data collection. It helps organizations respond quickly to adverse events, ultimately enhancing safety and operational efficiency.
-
Is the adverse event form template customizable?
Yes, the adverse event form template on airSlate SignNow is fully customizable. You can modify fields, add specific questions, and adjust the layout to suit your organization's requirements, ensuring that all relevant information is captured.
Get more for Serious Adverse Event Report Form SAE Form
Find out other Serious Adverse Event Report Form SAE Form
- eSignature New Jersey Healthcare / Medical Credit Memo Myself
- eSignature North Dakota Healthcare / Medical Medical History Simple
- Help Me With eSignature Arkansas High Tech Arbitration Agreement
- eSignature Ohio Healthcare / Medical Operating Agreement Simple
- eSignature Oregon Healthcare / Medical Limited Power Of Attorney Computer
- eSignature Pennsylvania Healthcare / Medical Warranty Deed Computer
- eSignature Texas Healthcare / Medical Bill Of Lading Simple
- eSignature Virginia Healthcare / Medical Living Will Computer
- eSignature West Virginia Healthcare / Medical Claim Free
- How To eSignature Kansas High Tech Business Plan Template
- eSignature Kansas High Tech Lease Agreement Template Online
- eSignature Alabama Insurance Forbearance Agreement Safe
- How Can I eSignature Arkansas Insurance LLC Operating Agreement
- Help Me With eSignature Michigan High Tech Emergency Contact Form
- eSignature Louisiana Insurance Rental Application Later
- eSignature Maryland Insurance Contract Safe
- eSignature Massachusetts Insurance Lease Termination Letter Free
- eSignature Nebraska High Tech Rental Application Now
- How Do I eSignature Mississippi Insurance Separation Agreement
- Help Me With eSignature Missouri Insurance Profit And Loss Statement