Fda Form 3514


What is the FDA Form 3514?
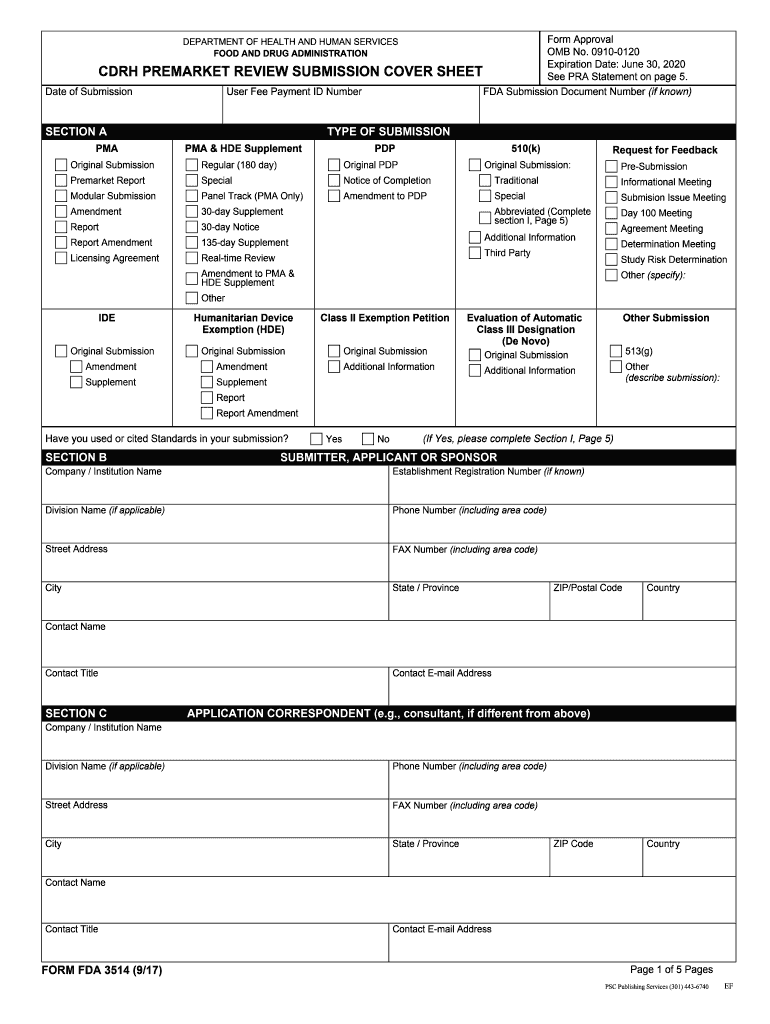
The FDA Form 3514 is a submission form used by the Food and Drug Administration (FDA) for various regulatory purposes. Specifically, it is often associated with applications for medical devices, ensuring that the products meet safety and efficacy standards before they can be marketed. This form is crucial for manufacturers seeking to gain approval for their devices, as it collects essential information regarding the product's design, intended use, and manufacturing processes.
How to Use the FDA Form 3514
Using the FDA Form 3514 involves several steps to ensure that all required information is accurately provided. First, gather all necessary documentation related to the device, including technical specifications, labeling, and any previous correspondence with the FDA. Next, complete the form by filling in all requested fields, ensuring that the information is clear and precise. After completing the form, review it for accuracy before submission. This thorough approach helps facilitate a smoother review process by the FDA.
Steps to Complete the FDA Form 3514
Completing the FDA Form 3514 requires careful attention to detail. Here are the key steps:
- Gather relevant documentation, including device specifications and labeling.
- Fill out the form, ensuring all fields are completed accurately.
- Provide a clear description of the device, including its intended use and any claims made.
- Review the form for completeness and accuracy.
- Submit the form along with any required fees and supporting documents.
Legal Use of the FDA Form 3514
The legal use of the FDA Form 3514 is governed by federal regulations that outline the requirements for medical device submissions. It is essential that all information provided is truthful and complies with FDA standards. Misrepresentation or omission of critical information can lead to penalties, including denial of the application or legal action. Therefore, it is vital to ensure that the form is filled out with the utmost care and in accordance with the law.
Key Elements of the FDA Form 3514
Several key elements must be included in the FDA Form 3514 to ensure a complete submission. These elements typically include:
- Device name and model number
- Manufacturer information
- Description of the device's intended use
- Details regarding the manufacturing process
- Any previous FDA correspondence related to the device
Form Submission Methods
The FDA Form 3514 can be submitted through various methods, depending on the specific requirements set forth by the FDA. Common submission methods include:
- Online submission through the FDA's electronic submission gateway
- Mailing a hard copy of the form to the appropriate FDA office
- In-person submission, if applicable, at designated FDA locations
Quick guide on how to complete fda form 3514
Complete Fda Form 3514 effortlessly on any device
Digital document management has gained signNow popularity among companies and individuals. It offers an excellent eco-friendly substitute for conventional printed and signed documents, enabling you to obtain the necessary form and securely save it online. airSlate SignNow equips you with all the resources needed to create, modify, and electronically sign your documents swiftly without any hassles. Manage Fda Form 3514 on any device using airSlate SignNow's Android or iOS applications and streamline any document-related task today.
How to modify and electronically sign Fda Form 3514 with ease
- Obtain Fda Form 3514 and click on Get Form to begin.
- Utilize the tools provided to fill out your form.
- Emphasize relevant sections of the documents or obscure sensitive information using the tools specifically designed for that purpose by airSlate SignNow.
- Create your electronic signature using the Sign tool, which takes mere seconds and holds the same legal validity as a conventional handwritten signature.
- Verify all the details and click on the Done button to save your modifications.
- Choose how you wish to submit your form, via email, text message (SMS), invitation link, or download it to your computer.
Eliminate concerns about lost or misplaced documents, tedious form searching, or mistakes that require new document copies. airSlate SignNow meets your requirements in document management with just a few clicks from any preferred device. Edit and eSign Fda Form 3514 and ensure excellent communication throughout your form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the fda form 3514
The best way to generate an eSignature for your PDF file in the online mode
The best way to generate an eSignature for your PDF file in Chrome
How to make an eSignature for putting it on PDFs in Gmail
How to create an electronic signature right from your smartphone
How to create an electronic signature for a PDF file on iOS devices
How to create an electronic signature for a PDF on Android
People also ask
-
What is FDA 3514 and how can airSlate SignNow help?
FDA 3514 refers to the regulatory framework established for certain document management processes in the healthcare sector. airSlate SignNow simplifies compliance by allowing businesses to securely eSign and manage their FDA 3514-related documents efficiently, ensuring that they meet all necessary FDA standards.
-
What features does airSlate SignNow offer for FDA 3514 compliance?
airSlate SignNow provides various features such as secure eSigning, document storage, and audit trails that are essential for FDA 3514 compliance. These tools help businesses maintain the integrity of their documents while ensuring they can easily track changes and maintain proper records.
-
Is airSlate SignNow cost-effective for businesses handling FDA 3514 documents?
Yes, airSlate SignNow offers a competitive pricing structure that is particularly cost-effective for businesses managing FDA 3514 documents. By streamlining the eSigning and document management processes, companies can reduce overhead costs while ensuring compliance with FDA regulations.
-
Can airSlate SignNow integrate with other platforms to facilitate FDA 3514 processes?
Absolutely! airSlate SignNow integrates seamlessly with various platforms such as CRM systems, cloud storage solutions, and productivity tools, which helps businesses manage their FDA 3514 processes more effectively. This integration ensures that all relevant data is easily accessible and workflows remain smooth.
-
How does airSlate SignNow ensure the security of FDA 3514 documents?
Security is a top priority for airSlate SignNow, especially for sensitive FDA 3514 documents. The platform uses advanced encryption methods and multi-factor authentication to protect data, ensuring that only authorized users can access critical information.
-
What are the benefits of using airSlate SignNow for FDA 3514 documentation?
Using airSlate SignNow for FDA 3514 documentation simplifies the signing process and enhances regulatory compliance. The platform reduces the time and effort required to prepare, sign, and manage documents, allowing businesses to focus more on their core operations rather than paperwork.
-
Is there support available for businesses using airSlate SignNow for FDA 3514 compliance?
Yes, airSlate SignNow provides dedicated customer support to assist businesses with their FDA 3514 compliance needs. Our knowledgeable support team is available to help clients navigate the platform and troubleshoot any issues related to their eSigning processes.
Get more for Fda Form 3514
Find out other Fda Form 3514
- Can I eSignature Nevada Non-disclosure agreement PDF
- eSignature New Mexico Non-disclosure agreement PDF Online
- Can I eSignature Utah Non-disclosure agreement PDF
- eSignature Rhode Island Rental agreement lease Easy
- eSignature New Hampshire Rental lease agreement Simple
- eSignature Nebraska Rental lease agreement forms Fast
- eSignature Delaware Rental lease agreement template Fast
- eSignature West Virginia Rental lease agreement forms Myself
- eSignature Michigan Rental property lease agreement Online
- Can I eSignature North Carolina Rental lease contract
- eSignature Vermont Rental lease agreement template Online
- eSignature Vermont Rental lease agreement template Now
- eSignature Vermont Rental lease agreement template Free
- eSignature Nebraska Rental property lease agreement Later
- eSignature Tennessee Residential lease agreement Easy
- Can I eSignature Washington Residential lease agreement
- How To eSignature Vermont Residential lease agreement form
- How To eSignature Rhode Island Standard residential lease agreement
- eSignature Mississippi Commercial real estate contract Fast
- eSignature Arizona Contract of employment Online