Fda Form 3514


What is the FDA Form 3514
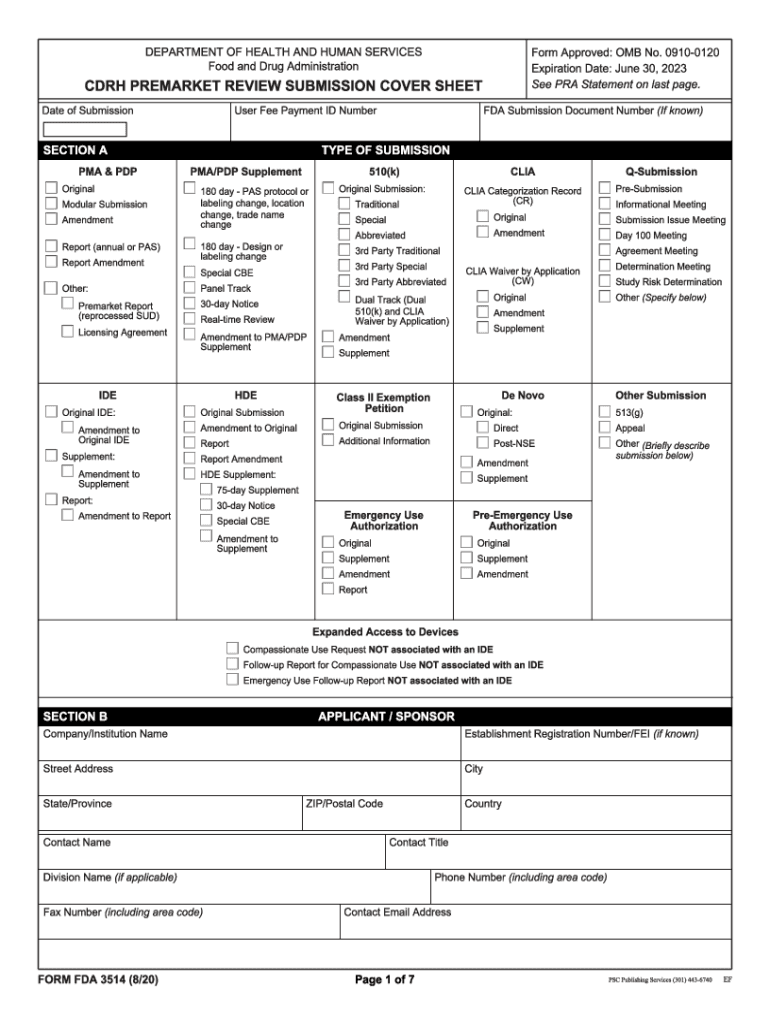
The FDA Form 3514, also known as the HHS submission cover sheet, is a critical document used in the premarket review process for medical devices. This form is required by the Center for Devices and Radiological Health (CDRH) within the FDA. It serves as a cover sheet for submissions, providing essential information about the device, the manufacturer, and the type of submission being made. The form helps streamline the review process and ensures that all necessary details are included for efficient evaluation.
How to Use the FDA Form 3514
Using the FDA Form 3514 involves several steps to ensure that all required information is accurately provided. First, gather all relevant details about the device, including its name, intended use, and any applicable regulatory history. Next, complete the form by filling in the required fields, such as the submitter's contact information and details about the submission type. Once completed, the form should be included with the submission package sent to the FDA for review. It is crucial to double-check all entries for accuracy to avoid delays in the review process.
Steps to Complete the FDA Form 3514
Completing the FDA Form 3514 requires careful attention to detail. Follow these steps:
- Download the form from the FDA website or obtain it through official channels.
- Fill in the submitter's information, including name, address, and contact details.
- Provide device-specific information, such as the device name, model number, and intended use.
- Indicate the type of submission, whether it is a premarket notification (510(k)), premarket approval (PMA), or other relevant categories.
- Review all entries for completeness and accuracy.
- Sign and date the form as required.
Legal Use of the FDA Form 3514
The FDA Form 3514 is legally binding when properly completed and submitted. It is essential to comply with all relevant regulations and guidelines set forth by the FDA to ensure that the submission is valid. The form must be filled out accurately to avoid potential legal issues or delays in the review process. Additionally, electronic submissions must adhere to the Electronic Signatures in Global and National Commerce (ESIGN) Act and other applicable laws to maintain their legal standing.
Key Elements of the FDA Form 3514
Several key elements must be included in the FDA Form 3514 to ensure it meets regulatory requirements:
- Submitter Information: Complete details about the manufacturer or submitter.
- Device Description: Clear identification of the device, including its name and intended use.
- Submission Type: Specification of whether the submission is a 510(k), PMA, or another type.
- Signatures: Required signatures from authorized individuals, confirming the accuracy of the information provided.
Form Submission Methods
The FDA Form 3514 can be submitted through various methods, depending on the submission type. Common methods include:
- Online Submission: Many submissions can be made electronically through the FDA's electronic submission gateway.
- Mail: Traditional paper submissions can be sent via postal mail to the appropriate FDA address.
- In-Person: In some cases, submissions may be delivered directly to FDA offices.
Quick guide on how to complete fda form 3514 568862806
Effortlessly Prepare Fda Form 3514 on Any Device
Digital document management has gained popularity among businesses and individuals. It offers an ideal eco-friendly alternative to conventional printed and signed paperwork, allowing you to obtain the correct form and securely store it online. airSlate SignNow provides you with all the necessary tools to create, modify, and eSign your documents swiftly without delays. Manage Fda Form 3514 on any platform using airSlate SignNow's Android or iOS applications and simplify any document-related process today.
How to Edit and eSign Fda Form 3514 with Ease
- Locate Fda Form 3514 and click on Get Form to begin.
- Utilize the tools we provide to complete your form.
- Highlight signNow sections of the documents or redact sensitive information using the tools that airSlate SignNow specifically offers for this purpose.
- Create your signature with the Sign tool, which takes seconds and holds the same legal authenticity as a traditional handwritten signature.
- Review all the details and click on the Done button to save your modifications.
- Choose how you wish to deliver your form, via email, text message (SMS), invite link, or download it to your computer.
Eliminate concerns about missing or lost files, tedious form navigation, or errors that necessitate printing new copies. airSlate SignNow addresses all your document management needs in just a few clicks from any device you prefer. Modify and eSign Fda Form 3514 to ensure excellent communication throughout the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the fda form 3514 568862806
The way to generate an eSignature for a PDF file in the online mode
The way to generate an eSignature for a PDF file in Chrome
How to create an electronic signature for putting it on PDFs in Gmail
The best way to generate an eSignature from your smartphone
The way to create an eSignature for a PDF file on iOS devices
The best way to generate an eSignature for a PDF file on Android
People also ask
-
What is the FDA Form 3514 and how does it relate to airSlate SignNow?
The FDA Form 3514 is a key document used for applications related to drug products. AirSlate SignNow simplifies the process of completing and submitting the FDA Form 3514 by providing a secure platform for electronic signatures and document management. This ensures compliance while saving time and resources.
-
How can I eSign the FDA Form 3514 using airSlate SignNow?
To eSign FDA Form 3514 with airSlate SignNow, simply upload the document to your dashboard, add the necessary fields for signatures, and invite the relevant parties to sign electronically. Our intuitive interface makes it easy to manage the signing process efficiently. This method enhances accuracy and reduces processing times.
-
What are the pricing options for using airSlate SignNow to handle FDA Form 3514?
AirSlate SignNow offers various pricing plans tailored to meet the needs of different businesses. Each plan provides access to features that facilitate the eSigning of important documents like the FDA Form 3514. You can choose a plan that best fits your operational requirements and budget.
-
What features make airSlate SignNow ideal for managing FDA Form 3514?
AirSlate SignNow includes features like customizable templates, secure cloud storage, and automated workflows that streamline the management of your FDA Form 3514. Additionally, our platform supports advanced security measures to protect sensitive data during the eSigning process, ensuring compliance with regulatory standards.
-
Can airSlate SignNow integrate with other software for FDA Form 3514 processing?
Yes, airSlate SignNow seamlessly integrates with various software platforms such as CRMs and document management systems to enhance your workflow related to the FDA Form 3514. This integration allows for a more organized approach by reducing manual entry and increasing efficiency in handling documents.
-
What are the benefits of using airSlate SignNow for electronic signatures on FDA Form 3514?
Utilizing airSlate SignNow for electronic signatures on FDA Form 3514 provides numerous benefits, including faster turnaround times and improved accuracy. Our platform is designed to reduce the paperwork burden, enabling teams to focus on critical tasks while ensuring that all signatures are legally binding and compliant.
-
Is there a mobile app available for signing FDA Form 3514?
Yes, airSlate SignNow offers a mobile app that allows you to sign the FDA Form 3514 on-the-go. This flexibility means that you can complete important tasks wherever you are, ensuring that the signing process does not delay your projects. Mobile access ensures convenience and efficiency.
Get more for Fda Form 3514
- Kifas application form
- Statens fellesblankett form
- Prushield form
- Tennessees individual education program iep form
- Application for temporary operation permit form
- Franchise and excise tax return form
- Form st 809 new york state and local sales and use tax return for part quarterly monthly filers revised 1024
- Instructions for form et 706 new york state estate tax return for an estate of an individual who died on or after january 1 769879895
Find out other Fda Form 3514
- How Can I eSignature Vermont Police Presentation
- How Do I eSignature Pennsylvania Real Estate Document
- How Do I eSignature Texas Real Estate Document
- How Can I eSignature Colorado Courts PDF
- Can I eSignature Louisiana Courts Document
- How To Electronic signature Arkansas Banking Document
- How Do I Electronic signature California Banking Form
- How Do I eSignature Michigan Courts Document
- Can I eSignature Missouri Courts Document
- How Can I Electronic signature Delaware Banking PDF
- Can I Electronic signature Hawaii Banking Document
- Can I eSignature North Carolina Courts Presentation
- Can I eSignature Oklahoma Courts Word
- How To Electronic signature Alabama Business Operations Form
- Help Me With Electronic signature Alabama Car Dealer Presentation
- How Can I Electronic signature California Car Dealer PDF
- How Can I Electronic signature California Car Dealer Document
- How Can I Electronic signature Colorado Car Dealer Form
- How To Electronic signature Florida Car Dealer Word
- How Do I Electronic signature Florida Car Dealer Document