Human Cells, Tissues and Organs for Transplantation Adverse Reaction Reporting Form Version 7 0


What is the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0
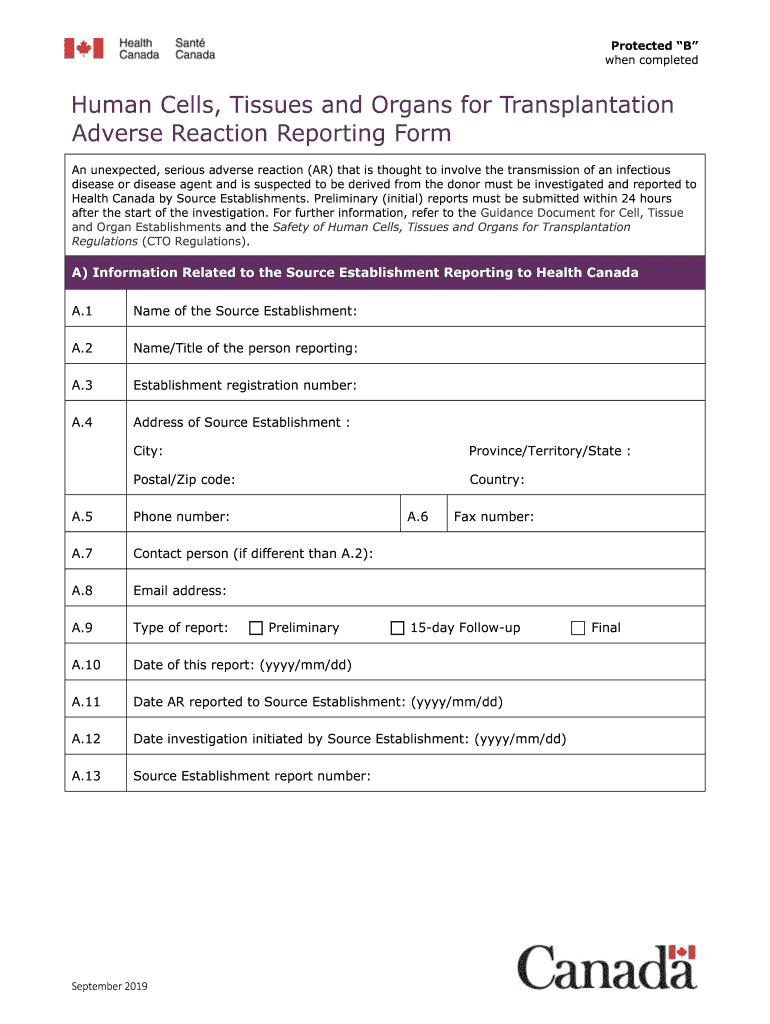
The Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0 is a crucial document designed for reporting adverse reactions related to the use of human cells, tissues, and organs in transplantation procedures. This form is essential for healthcare providers and institutions to ensure patient safety and regulatory compliance. By documenting adverse reactions, the form helps in monitoring the safety and efficacy of transplant procedures, contributing to better healthcare outcomes.
Steps to complete the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0
Completing the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0 involves several key steps:
- Gather all necessary patient information, including demographics and medical history.
- Document the specific adverse reaction experienced, detailing the nature and severity.
- Include information about the transplant procedure, such as the type of tissue or organ used.
- Provide details on the date of the reaction and any follow-up actions taken.
- Review the completed form for accuracy and completeness before submission.
How to use the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0
The use of the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0 is straightforward. Users can fill out the form electronically or on paper. When using the electronic version, ensure that all fields are completed accurately. After filling out the form, it can be submitted through the appropriate channels, which may include online submission or mailing to the designated authority. Utilizing electronic signatures can enhance the submission process, making it more efficient and secure.
Legal use of the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0
The legal use of the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0 is governed by various regulations that ensure the form's validity and compliance. For the form to be legally binding, it must be completed accurately and submitted to the appropriate regulatory bodies. Compliance with eSignature laws, such as the ESIGN Act and UETA, is essential when submitting the form electronically, ensuring that all signatures are recognized as legally valid.
Key elements of the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0
Key elements of the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0 include:
- Patient identification information, including name and medical record number.
- Details of the adverse reaction, including symptoms and onset.
- Information about the transplant procedure, including the type of organ or tissue.
- Contact information for the reporting healthcare provider.
- Signature of the reporting individual, confirming the accuracy of the information provided.
How to obtain the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0
The Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0 can be obtained from regulatory agencies or healthcare organizations involved in transplantation. Many institutions provide access to the form through their websites, allowing users to download or fill it out electronically. It is essential to ensure that the most current version of the form is used to comply with the latest reporting standards.
Quick guide on how to complete human cells tissues and organs for transplantation adverse reaction reporting form version 70
Effortlessly Prepare Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0 on Any Device
Digital document management has become increasingly popular among both enterprises and individuals. It serves as an ideal eco-friendly substitute for conventional printed and signed documents, as you can obtain the necessary form and securely archive it online. airSlate SignNow provides you with all the tools required to create, modify, and electronically sign your documents promptly without delays. Handle Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0 on any device using the airSlate SignNow Android or iOS applications and simplify any document-related task today.
The Easiest Way to Modify and Electronically Sign Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0 with Ease
- Access Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0 and click Get Form to begin.
- Utilize the tools we offer to fill out your form.
- Emphasize important sections of your documents or redact sensitive details with the tools that airSlate SignNow provides specifically for that purpose.
- Create your electronic signature with the Sign tool, which only takes a few seconds and has the same legal validity as a traditional handwritten signature.
- Review all the information and click the Done button to save your modifications.
- Select how you want to share your form, whether by email, text message (SMS), invitation link, or download it to your computer.
Forget about lost or misplaced documents, tedious searches for forms, or errors that require reprinting new copies. airSlate SignNow meets your document management needs in just a few clicks from any device you choose. Edit and electronically sign Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0 to ensure excellent communication at every stage of your form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
People also ask
-
What is the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0?
The Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0 is a specific document designed for reporting adverse reactions related to the transplantation of human cells, tissues, and organs. This form helps ensure compliance with regulatory standards while streamlining the reporting process for healthcare providers.
-
How does airSlate SignNow support the completion of the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0?
airSlate SignNow provides a user-friendly platform that facilitates easy completion and electronic signing of the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0. Users can quickly fill out the form and send it for approval, ensuring prompt reporting and compliance.
-
Are there any costs associated with using the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0 on airSlate SignNow?
Yes, there may be subscription costs related to using airSlate SignNow for the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0. However, the platform offers various pricing plans that cater to different business sizes and needs, ensuring a cost-effective solution.
-
What features does airSlate SignNow provide for the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0?
airSlate SignNow offers features such as digital signatures, document templates, and secure cloud storage for the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0. These features simplify the process of managing and submitting important documents while maintaining security and compliance.
-
How can I integrate airSlate SignNow with other software for processing the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0?
airSlate SignNow provides integrations with various software platforms, making it easy to incorporate the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0 into your existing workflows. You can connect it with practice management systems, CRMs, and more to streamline your operations.
-
What are the benefits of using airSlate SignNow for the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0?
Using airSlate SignNow for the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0 enhances efficiency, reduces paperwork, and supports regulatory compliance. The platform's eSigning capabilities also speed up the approval process, ensuring timely reporting of adverse reactions.
-
Is there a mobile app for completing the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0 on the go?
Yes, airSlate SignNow offers a mobile app that allows users to complete and sign the Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0 from their smartphones or tablets. This mobile flexibility ensures that healthcare providers can manage important documentation anytime and anywhere.
Get more for Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0
- Oregon letter 497323677 form
- Letter from landlord to tenant where tenant complaint was caused by the deliberate or negligent act of tenant or tenants guest 497323678 form
- Letter from landlord to tenant for failure to keep premises as clean and safe as condition of premises permits remedy or lease 497323679 form
- Letter from landlord to tenant for failure of to dispose all ashes rubbish garbage or other waste in a clean and safe manner in 497323680 form
- Letter from landlord to tenant for failure to keep all plumbing fixtures in the dwelling unit as clean as their condition 497323681 form
- Oregon landlord in form
- Letter from landlord to tenant as notice to tenant of tenants disturbance of neighbors peaceful enjoyment to remedy or lease 497323683 form
- Oregon landlord notice form
Find out other Human Cells, Tissues And Organs For Transplantation Adverse Reaction Reporting Form Version 7 0
- Sign South Dakota Charity Residential Lease Agreement Simple
- Sign Vermont Charity Business Plan Template Later
- Sign Arkansas Construction Executive Summary Template Secure
- How To Sign Arkansas Construction Work Order
- Sign Colorado Construction Rental Lease Agreement Mobile
- Sign Maine Construction Business Letter Template Secure
- Can I Sign Louisiana Construction Letter Of Intent
- How Can I Sign Maryland Construction Business Plan Template
- Can I Sign Maryland Construction Quitclaim Deed
- Sign Minnesota Construction Business Plan Template Mobile
- Sign Construction PPT Mississippi Myself
- Sign North Carolina Construction Affidavit Of Heirship Later
- Sign Oregon Construction Emergency Contact Form Easy
- Sign Rhode Island Construction Business Plan Template Myself
- Sign Vermont Construction Rental Lease Agreement Safe
- Sign Utah Construction Cease And Desist Letter Computer
- Help Me With Sign Utah Construction Cease And Desist Letter
- Sign Wisconsin Construction Purchase Order Template Simple
- Sign Arkansas Doctors LLC Operating Agreement Free
- Sign California Doctors Lease Termination Letter Online