FORM FDA 3674 Certification of Compliance under 42 U S C 282j5B, with Requirements of ClinicalTrials Gov Data Bank 2019


Understanding the FDA Form 3674 Certification of Compliance
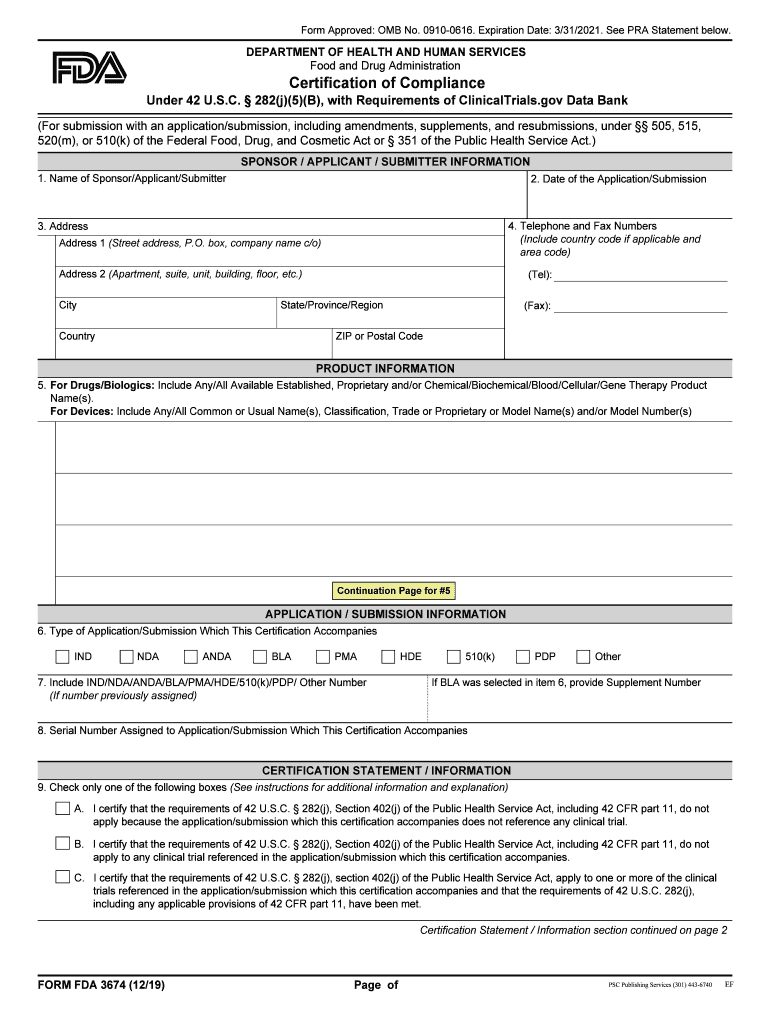
The FDA Form 3674 is a Certification of Compliance under 42 U.S.C. 282j5B, which is essential for entities involved in clinical trials. This form ensures that the necessary data is submitted to the ClinicalTrials.gov data bank, confirming adherence to federal regulations. The certification is crucial for maintaining transparency and accountability in clinical research, as it verifies that the information provided aligns with legal requirements and ethical standards.
Steps to Complete the FDA Form 3674
Completing the FDA Form 3674 involves several key steps to ensure accuracy and compliance. Begin by gathering all necessary information regarding the clinical trial, including the trial's title, purpose, and responsible party details. Next, fill out the form with precise data, ensuring that all sections are completed according to the guidelines provided by the FDA. After filling out the form, review it thoroughly for any errors or omissions. Finally, submit the form electronically through the designated platform, ensuring that you receive confirmation of submission for your records.
Legal Use of the FDA Form 3674
The legal use of the FDA Form 3674 is governed by federal regulations that mandate compliance with clinical trial reporting requirements. This form serves as a legal document, affirming that the submitting entity has met all criteria set forth by the FDA. Failure to submit this form or providing inaccurate information can result in penalties, including fines or restrictions on conducting clinical trials. Therefore, it is crucial to understand the legal implications and ensure that the form is completed accurately and submitted on time.
Key Elements of the FDA Form 3674
Key elements of the FDA Form 3674 include the identification of the responsible party, the title of the clinical trial, and the specific compliance statements required under 42 U.S.C. 282j5B. Each section of the form is designed to capture essential information that reflects the trial's adherence to reporting standards. Additionally, the form requires the submission of information about the trial's results, ensuring that all data is made publicly available in accordance with federal law.
Obtaining the FDA Form 3674
The FDA Form 3674 can be obtained directly from the FDA's official website or through the ClinicalTrials.gov platform. It is important to ensure that you are using the most current version of the form, as regulations and requirements may change. Accessing the form online allows for easy completion and submission, streamlining the process for researchers and institutions involved in clinical trials.
Examples of Using the FDA Form 3674
Examples of using the FDA Form 3674 include its application in various clinical trials across different medical fields. For instance, a pharmaceutical company conducting a trial for a new drug must submit this form to certify compliance with federal regulations. Similarly, academic institutions involved in clinical research must also utilize the form to ensure that their trials are reported accurately. These examples highlight the form's role in promoting transparency and accountability in clinical research practices.
Quick guide on how to complete form fda 3674 certification of compliance under 42 u s c 282j5b with requirements of clinicaltrials gov data bank
Accomplish FORM FDA 3674 Certification Of Compliance Under 42 U S C 282j5B, With Requirements Of ClinicalTrials gov Data Bank effortlessly on any device
Digital document management has gained signNow traction among businesses and individuals. It offers an ideal eco-friendly substitute for traditional printed and signed documents, as you can access the appropriate form and securely save it online. airSlate SignNow provides you with all the tools needed to create, modify, and electronically sign your documents quickly without delays. Manage FORM FDA 3674 Certification Of Compliance Under 42 U S C 282j5B, With Requirements Of ClinicalTrials gov Data Bank on any device using the airSlate SignNow Android or iOS applications and streamline any document-related process today.
How to modify and electronically sign FORM FDA 3674 Certification Of Compliance Under 42 U S C 282j5B, With Requirements Of ClinicalTrials gov Data Bank with ease
- Obtain FORM FDA 3674 Certification Of Compliance Under 42 U S C 282j5B, With Requirements Of ClinicalTrials gov Data Bank and click Get Form to initiate the process.
- Utilize the tools we provide to complete your form.
- Emphasize important sections of the documents or redact sensitive information with tools that airSlate SignNow specifically offers for this purpose.
- Create your electronic signature with the Sign tool, which takes just seconds and has the same legal validity as a conventional handwritten signature.
- Review all the details and click the Done button to save your modifications.
- Select your preferred method to send your form, whether by email, SMS, invite link, or download it to your computer.
Say goodbye to lost or misplaced files, tedious form searches, or errors that require reprinting new document copies. airSlate SignNow addresses all your document management needs in just a few clicks from any device you choose. Edit and electronically sign FORM FDA 3674 Certification Of Compliance Under 42 U S C 282j5B, With Requirements Of ClinicalTrials gov Data Bank to ensure excellent communication at every stage of the document preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Find and fill out the correct form fda 3674 certification of compliance under 42 u s c 282j5b with requirements of clinicaltrials gov data bank
Create this form in 5 minutes!
How to create an eSignature for the form fda 3674 certification of compliance under 42 u s c 282j5b with requirements of clinicaltrials gov data bank
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is the significance of the FDA 3674 form in document eSigning?
The FDA 3674 form is crucial for compliance, especially in the healthcare industry. It ensures that electronic signatures are used in accordance with FDA regulations. By using airSlate SignNow for your eSigning needs, you can easily manage and submit FDA 3674 forms securely.
-
How does airSlate SignNow simplify the process of signing FDA 3674 forms?
airSlate SignNow simplifies the signing process by offering an intuitive interface for all users. You can quickly upload the FDA 3674 form, add necessary signers, and track the status of each signature seamlessly. This helps eliminate paperwork and speeds up your compliance processes.
-
What are the pricing options for using airSlate SignNow for FDA 3674 eSignatures?
airSlate SignNow offers flexible pricing plans tailored to different business needs. You can choose a plan that fits your budget while still ensuring that you have the capabilities to handle FDA 3674 eSignatures efficiently. Enhanced features are available in premium plans for businesses requiring additional functionality.
-
Can airSlate SignNow integrate with other platforms for handling FDA 3674 documents?
Yes, airSlate SignNow supports integration with various applications, enabling you to streamline your workflow for managing FDA 3674 documents. Whether you need to connect with CRMs, cloud storage, or other business tools, our platform ensures a seamless experience, making document management efficient.
-
What features does airSlate SignNow offer for secure signing of FDA 3674 forms?
Security is a priority for airSlate SignNow, especially when dealing with sensitive documents like FDA 3674 forms. Our platform offers robust encryption and audit trails to ensure the integrity and confidentiality of your documents, allowing businesses to meet regulatory requirements confidently.
-
How can airSlate SignNow benefit businesses dealing with FDA 3674 compliance?
By utilizing airSlate SignNow, businesses can speed up their compliance processes related to FDA 3674 forms. The platform allows for rapid eSigning and real-time tracking, reducing the administrative burden and ensuring that your organization meets FDA requirements effectively.
-
Are there any mobile solutions available for signing FDA 3674 documents with airSlate SignNow?
Yes, airSlate SignNow provides a mobile application that allows you to sign FDA 3674 documents on the go. This means you can manage your eSigning tasks from anywhere, ensuring you never miss the opportunity to complete necessary compliance documentation quickly and efficiently.
Get more for FORM FDA 3674 Certification Of Compliance Under 42 U S C 282j5B, With Requirements Of ClinicalTrials gov Data Bank
- Letter tenant landlord repair form
- Letter from tenant to landlord with demand that landlord remove garbage and vermin from premises maine form
- Letter from tenant to landlord with demand that landlord provide proper outdoor garbage receptacles maine form
- Letter from tenant to landlord about landlords failure to make repairs maine form
- Letter from landlord to tenant as notice that rent was voluntarily lowered in exchange for tenant agreeing to make repairs 497310784 form
- Letter from tenant to landlord about landlord using unlawful self help to gain possession maine form
- Letter from tenant to landlord about illegal entry by landlord maine form
- Letter from landlord to tenant about time of intent to enter premises maine form
Find out other FORM FDA 3674 Certification Of Compliance Under 42 U S C 282j5B, With Requirements Of ClinicalTrials gov Data Bank
- eSign Education PDF Wyoming Mobile
- Can I eSign Nebraska Finance & Tax Accounting Business Plan Template
- eSign Nebraska Finance & Tax Accounting Business Letter Template Online
- eSign Nevada Finance & Tax Accounting Resignation Letter Simple
- eSign Arkansas Government Affidavit Of Heirship Easy
- eSign California Government LLC Operating Agreement Computer
- eSign Oklahoma Finance & Tax Accounting Executive Summary Template Computer
- eSign Tennessee Finance & Tax Accounting Cease And Desist Letter Myself
- eSign Finance & Tax Accounting Form Texas Now
- eSign Vermont Finance & Tax Accounting Emergency Contact Form Simple
- eSign Delaware Government Stock Certificate Secure
- Can I eSign Vermont Finance & Tax Accounting Emergency Contact Form
- eSign Washington Finance & Tax Accounting Emergency Contact Form Safe
- How To eSign Georgia Government Claim
- How Do I eSign Hawaii Government Contract
- eSign Hawaii Government Contract Now
- Help Me With eSign Hawaii Government Contract
- eSign Hawaii Government Contract Later
- Help Me With eSign California Healthcare / Medical Lease Agreement
- Can I eSign California Healthcare / Medical Lease Agreement