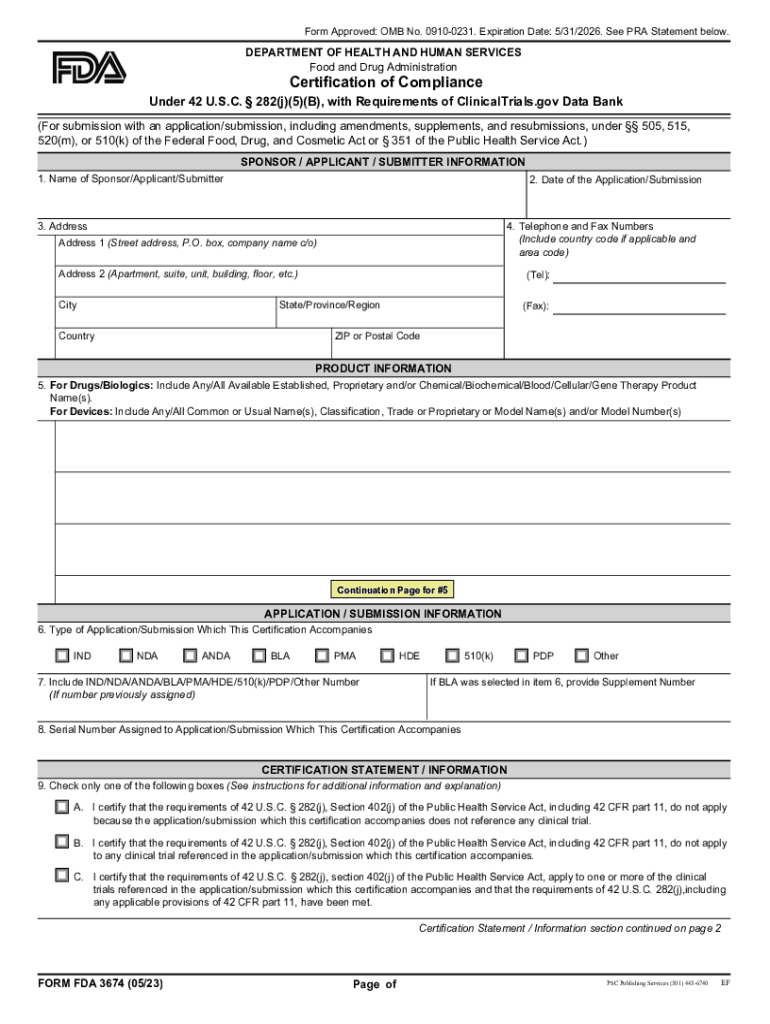
FORM FDA 3674 Certification of Compliance under 42 U S C 282j5B, with Requirements of ClinicalTrials Gov Data Bank 2023


Understanding the FORM FDA 3674 Certification of Compliance
The FORM FDA 3674, known as the Certification of Compliance Under 42 U.S.C. 282(j)(5)(B), is a crucial document for entities involved in clinical trials. This form certifies that the entity complies with the requirements set forth by the ClinicalTrials.gov Data Bank. It is essential for ensuring transparency and accountability in clinical research, as it mandates the registration and results reporting of clinical trials conducted in the United States. By submitting this form, researchers affirm their commitment to ethical standards and regulatory compliance.
How to Complete the FORM FDA 3674
Filling out the FORM FDA 3674 involves several key steps. First, ensure you have all necessary information regarding the clinical trial, including the trial's title, registration number, and relevant dates. Next, accurately complete each section of the form, providing detailed information about compliance with ClinicalTrials.gov requirements. It is important to review the form for accuracy before submission. Once completed, the form can be submitted electronically or via mail, depending on the specific instructions provided by the FDA.
Obtaining the FORM FDA 3674
The FORM FDA 3674 can be obtained directly from the FDA's official website. It is available as a fillable PDF, allowing users to complete the form digitally. To download the form, navigate to the appropriate section of the FDA website, where you can access the latest version of the form. Ensure that you are using the most current version to avoid any compliance issues.
Key Elements of the FORM FDA 3674
Several critical elements must be included in the FORM FDA 3674. These include the name of the clinical trial, the principal investigator's details, and the organization conducting the trial. Additionally, the form requires confirmation that the trial data has been submitted to ClinicalTrials.gov, along with any relevant identifiers. Properly addressing these elements is vital for the form's acceptance and to maintain compliance with federal regulations.
Legal Implications of the FORM FDA 3674
The FORM FDA 3674 carries significant legal implications for researchers and organizations conducting clinical trials. Failure to submit this form or to comply with the reporting requirements can lead to penalties, including fines and restrictions on future research activities. It is essential for entities to understand the legal responsibilities associated with this form to avoid potential legal repercussions.
Submission Methods for the FORM FDA 3674
There are multiple methods for submitting the FORM FDA 3674. Researchers can choose to submit the form electronically through the FDA's designated online portal, which is often the preferred method due to its efficiency. Alternatively, the form can be mailed to the appropriate FDA office. When submitting by mail, it is advisable to use a trackable mailing option to ensure the form is received. Always check for the latest submission guidelines to ensure compliance.
Quick guide on how to complete form fda 3674 certification of compliance under 42 u s c 282j5b with requirements of clinicaltrials gov data bank 551806341
Complete FORM FDA 3674 Certification Of Compliance Under 42 U S C 282j5B, With Requirements Of ClinicalTrials gov Data Bank effortlessly on any device
Digital document management has become increasingly favored by organizations and individuals alike. It serves as an ideal eco-friendly substitute for traditional printed and signed documents, allowing you to locate the necessary form and securely save it online. airSlate SignNow equips you with all the features needed to create, edit, and electronically sign your documents swiftly without any delays. Handle FORM FDA 3674 Certification Of Compliance Under 42 U S C 282j5B, With Requirements Of ClinicalTrials gov Data Bank on any device using airSlate SignNow's Android or iOS applications and streamline any document-related task today.
The easiest way to modify and eSign FORM FDA 3674 Certification Of Compliance Under 42 U S C 282j5B, With Requirements Of ClinicalTrials gov Data Bank without stress
- Obtain FORM FDA 3674 Certification Of Compliance Under 42 U S C 282j5B, With Requirements Of ClinicalTrials gov Data Bank and click on Get Form to begin.
- Use the tools we provide to complete your form.
- Mark important sections of your documents or conceal sensitive information with tools specifically designed for that purpose by airSlate SignNow.
- Create your signature using the Sign feature, which takes mere seconds and carries the same legal validity as a traditional handwritten signature.
- Review all the details and click on the Done button to save your changes.
- Choose your preferred method to submit your form, whether by email, text message (SMS), invitation link, or download it to your computer.
Say goodbye to lost or misplaced documents, tedious form searches, or mistakes that necessitate printing new copies. airSlate SignNow meets your document management needs with just a few clicks from any device of your choice. Alter and eSign FORM FDA 3674 Certification Of Compliance Under 42 U S C 282j5B, With Requirements Of ClinicalTrials gov Data Bank to ensure smooth communication throughout the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Find and fill out the correct form fda 3674 certification of compliance under 42 u s c 282j5b with requirements of clinicaltrials gov data bank 551806341
Create this form in 5 minutes!
How to create an eSignature for the form fda 3674 certification of compliance under 42 u s c 282j5b with requirements of clinicaltrials gov data bank 551806341
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is the FDA 3674 form and why is it important?
The FDA 3674 form is a crucial document used in the regulation of clinical trials conducted in the United States. It ensures that necessary information is submitted to the FDA to comply with federal regulations. Understanding and effectively managing the FDA 3674 form is essential for any organization involved in drug research and development.
-
How can airSlate SignNow help with the FDA 3674 form?
airSlate SignNow offers an efficient solution for electronically signing and managing documents like the FDA 3674 form. With its user-friendly interface, you can easily prepare, send, and receive signatures for this essential form. This streamlines the compliance process and reduces the risk of errors.
-
What features does airSlate SignNow provide for handling the FDA 3674 form?
airSlate SignNow provides a variety of features tailored for handling documents like the FDA 3674 form, including secure eSigning, customizable templates, and automated workflows. These features help ensure timely submission and compliance with FDA regulations. The platform also offers real-time tracking, allowing you to monitor the status of your documents.
-
Is airSlate SignNow cost-effective for managing the FDA 3674 form?
Yes, airSlate SignNow is a cost-effective solution for managing documents like the FDA 3674 form. It offers competitive pricing plans that cater to businesses of all sizes, helping you save money on physical document management and mailing costs. By digitizing your workflow, you can also reduce delays and improve efficiency.
-
Can I integrate airSlate SignNow with other software for the FDA 3674 form?
Absolutely! airSlate SignNow supports integrations with numerous software applications such as CRMs, project management tools, and cloud storage services. This allows you to streamline your process for the FDA 3674 form by connecting it with your existing workflows and data sources.
-
What are the benefits of using airSlate SignNow for the FDA 3674 form?
Using airSlate SignNow for the FDA 3674 form offers multiple benefits, including enhanced security, easy collaboration, and better document management. You can ensure compliance with FDA requirements while making the signing process faster and more reliable. This leads to a smoother experience for all parties involved.
-
Is it easy to use airSlate SignNow for new users handling the FDA 3674 form?
Yes, airSlate SignNow is designed for ease of use, making it accessible for new users unfamiliar with the FDA 3674 form. The platform provides intuitive navigation, helpful tutorials, and customer support to assist you in getting started. This simplicity allows you to focus on completing important documents quickly.
Get more for FORM FDA 3674 Certification Of Compliance Under 42 U S C 282j5B, With Requirements Of ClinicalTrials gov Data Bank
- Wb 40 amendment to offer to purchase 2009 form
- Offer to purchase real estate form winnebago county il 2006
- Wb 12 farm offer to purchase 1999 form
- Wisconsin real estate condition report form
- Landlordtenant guide from the wisconsin department of form
- Missouri sellers disclosure form
- Greater capital area association dc lease 2008 form
- Pg county sample tenant landlord agreement 2006 form
Find out other FORM FDA 3674 Certification Of Compliance Under 42 U S C 282j5B, With Requirements Of ClinicalTrials gov Data Bank
- How To Integrate Sign in Banking
- How To Use Sign in Banking
- Help Me With Use Sign in Banking
- Can I Use Sign in Banking
- How Do I Install Sign in Banking
- How To Add Sign in Banking
- How Do I Add Sign in Banking
- How Can I Add Sign in Banking
- Can I Add Sign in Banking
- Help Me With Set Up Sign in Government
- How To Integrate eSign in Banking
- How To Use eSign in Banking
- How To Install eSign in Banking
- How To Add eSign in Banking
- How To Set Up eSign in Banking
- How To Save eSign in Banking
- How To Implement eSign in Banking
- How To Set Up eSign in Construction
- How To Integrate eSign in Doctors
- How To Use eSign in Doctors