CONSORT Checklist of Items to Include When Reporting a Randomized Trial Product Name Form


What is the CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name
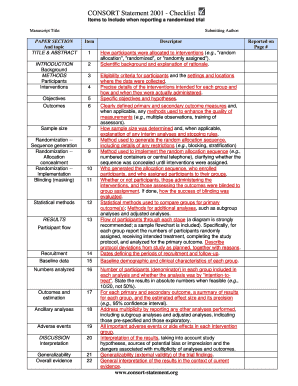
The CONSORT Checklist is a comprehensive tool designed to enhance the transparency and quality of reporting in randomized trials. It outlines essential items that researchers should include when documenting their study, ensuring that critical information is conveyed clearly. This checklist serves as a guideline for authors, reviewers, and editors to facilitate the accurate interpretation of trial results.
The checklist typically includes sections on trial design, participant flow, outcomes, and statistical methods, among others. By adhering to these standards, researchers can improve the reproducibility of their findings and contribute to the overall integrity of clinical research.
Key elements of the CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name
Understanding the key elements of the CONSORT Checklist is vital for effective reporting. The checklist generally comprises the following components:
- Title and abstract: Clear and concise presentation of the study's purpose and main findings.
- Introduction: Background information and rationale for the trial.
- Methods: Detailed description of the trial design, participants, interventions, and outcomes.
- Results: Comprehensive reporting of participant flow, outcomes, and statistical analyses.
- Discussion: Interpretation of results, limitations, and implications for practice.
These elements are crucial for ensuring that the study is reported in a manner that is both informative and accessible to readers.
How to use the CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name
Using the CONSORT Checklist effectively involves several steps. Researchers should begin by familiarizing themselves with the checklist items relevant to their specific trial. Each item should be addressed in the manuscript, with clear explanations and justifications provided where necessary.
As researchers draft their reports, they can use the checklist as a guide to ensure that no critical information is omitted. It is beneficial to review the checklist during the writing process and before submission to a journal. This practice can help enhance the quality of the manuscript and increase the likelihood of acceptance for publication.
Steps to complete the CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name
Completing the CONSORT Checklist involves a systematic approach. Here are the essential steps:
- Review the checklist: Obtain the latest version of the CONSORT Checklist and familiarize yourself with its items.
- Draft your report: Write each section of your manuscript, ensuring that you address all relevant checklist items.
- Cross-check: Use the checklist to verify that each item has been adequately covered in your report.
- Seek feedback: Consider sharing your draft with colleagues or mentors for additional insights.
- Revise: Make necessary adjustments based on feedback and ensure compliance with the checklist.
Following these steps can help streamline the reporting process and enhance the clarity of your trial results.
Legal use of the CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name
The legal implications of using the CONSORT Checklist are significant, especially in the context of clinical trials. Adhering to the checklist can help ensure compliance with ethical standards and regulatory requirements governing research. Proper reporting can safeguard against potential legal challenges related to data integrity and participant safety.
Moreover, many journals and institutions require adherence to the CONSORT guidelines as part of their submission criteria. This requirement underscores the importance of using the checklist to enhance the legal validity of trial reports.
Quick guide on how to complete consort checklist of items to include when reporting a randomized trial product name
Effortlessly Prepare CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name on Any Device
The management of documents online has become increasingly favored by enterprises and individuals. It serves as an excellent environmentally friendly alternative to traditional printed and signed documents, as you can easily obtain the correct form and securely save it online. airSlate SignNow provides all the tools necessary to create, modify, and electronically sign your documents quickly without delays. Handle CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name on any device with airSlate SignNow’s Android or iOS applications and enhance any document-centric process today.
How to Modify and Electronically Sign CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name with Ease
- Find CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name and click on Get Form to begin.
- Make use of the tools we provide to fill out your document.
- Highlight important sections of your documents or redact sensitive information using the tools specifically offered by airSlate SignNow.
- Create your signature using the Sign feature, which takes seconds and holds the same legal authority as a conventional wet ink signature.
- Review the details and click on the Done button to save your modifications.
- Choose your preferred method to send your form, whether by email, SMS, or shareable link, or download it to your computer.
Eliminate concerns about lost or mislaid documents, tedious form searching, or errors that necessitate printing new document copies. airSlate SignNow fulfills all your document management requirements in a few clicks from any device you prefer. Edit and electronically sign CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name while ensuring excellent communication at every stage of the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the consort checklist of items to include when reporting a randomized trial product name
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is the CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name?
The CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name is a vital tool that outlines essential components required for reporting randomized trials. It ensures transparency and completeness in trial reporting, facilitating peer review and enhancing the clarity of the research methodology.
-
How can airSlate SignNow assist in submitting the CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name?
With airSlate SignNow, you can easily prepare, sign, and submit trial documentation including the CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name. Our platform simplifies document handling and lets you organize your submission process efficiently, ensuring that all items are completed as required.
-
What are the pricing options for airSlate SignNow?
airSlate SignNow offers various pricing tiers to accommodate different business sizes and needs. Whether you’re a small startup or a large organization, you can find a plan that supports your document management, including the use of the CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name, at an affordable rate.
-
What features does airSlate SignNow provide for document management?
airSlate SignNow provides a range of features designed for effective document management, such as eSignature capabilities, secure cloud storage, and templates for compliance. These tools help streamline the process of adhering to standards like the CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name.
-
Are there integrations available with other software for using the CONSORT Checklist?
Yes, airSlate SignNow integrates seamlessly with various software applications, enhancing your workflow efficiency when using the CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name. This means you can connect your favorite tools and easily manage your documentation and signing processes.
-
What benefits can airSlate SignNow bring to trial reporting?
airSlate SignNow enhances trial reporting by providing a user-friendly interface to manage documents digitally, reducing time spent on paperwork. By facilitating compliance with the CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name, it helps ensure that all necessary components of the trial are accurately reported.
-
Is airSlate SignNow secure for handling sensitive trial documents?
Absolutely. airSlate SignNow employs robust security measures to ensure the protection of your sensitive trial documents, including those related to the CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name. Our platform includes encryption, secure access controls, and audit trails to maintain data integrity.
Get more for CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name
- Fedex packaging test application form
- Wayne county interim support order form
- Nys division of cemeteries annual report form
- Av request form equipment setup for presentations meetings kcpublicschools
- Esb staff services form
- University of wisconsin superior transcript request form
- Contrato de apertura de credito en cuenta corriente form
- Medical form staten island jcc summer camp camp sijcc
Find out other CONSORT Checklist Of Items To Include When Reporting A Randomized Trial Product Name
- Sign Delaware Insurance Claim Online
- Sign Delaware Insurance Contract Later
- Sign Hawaii Insurance NDA Safe
- Sign Georgia Insurance POA Later
- How Can I Sign Alabama Lawers Lease Agreement
- How Can I Sign California Lawers Lease Agreement
- Sign Colorado Lawers Operating Agreement Later
- Sign Connecticut Lawers Limited Power Of Attorney Online
- Sign Hawaii Lawers Cease And Desist Letter Easy
- Sign Kansas Insurance Rental Lease Agreement Mobile
- Sign Kansas Insurance Rental Lease Agreement Free
- Sign Kansas Insurance Rental Lease Agreement Fast
- Sign Kansas Insurance Rental Lease Agreement Safe
- How To Sign Kansas Insurance Rental Lease Agreement
- How Can I Sign Kansas Lawers Promissory Note Template
- Sign Kentucky Lawers Living Will Free
- Sign Kentucky Lawers LLC Operating Agreement Mobile
- Sign Louisiana Lawers Quitclaim Deed Now
- Sign Massachusetts Lawers Quitclaim Deed Later
- Sign Michigan Lawers Rental Application Easy