CONSORT Checklist of Information to Include When Reporting a Randomized Trial a


What is the CONSORT Checklist of Information to Include When Reporting a Randomized Trial A
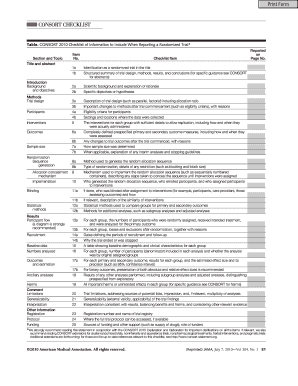
The CONSORT Checklist is a comprehensive tool designed to improve the transparency and quality of reporting in randomized trials. It outlines essential information that should be included when documenting the methodology and findings of a trial. This checklist serves as a guideline for researchers, ensuring that all critical elements are addressed, which helps in evaluating the validity and reliability of the trial results. By following the CONSORT Checklist, researchers can enhance the clarity of their reports, making it easier for readers to understand the study's design, execution, and outcomes.
How to Use the CONSORT Checklist of Information to Include When Reporting a Randomized Trial A
Using the CONSORT Checklist involves a systematic approach to reporting randomized trials. Researchers should begin by reviewing each item on the checklist and ensuring that their report addresses these points. This includes detailing the trial's design, participants, interventions, outcomes, and statistical analyses. As researchers draft their reports, they should refer back to the checklist to confirm that all necessary information is included. This practice not only aids in producing a comprehensive report but also facilitates peer review and publication processes.
Key Elements of the CONSORT Checklist of Information to Include When Reporting a Randomized Trial A
The key elements of the CONSORT Checklist encompass various aspects of the trial. These include:
- Title and abstract: A clear title that includes the study design and a structured abstract summarizing the key findings.
- Introduction: Background information explaining the rationale for the trial and its objectives.
- Methods: Detailed description of the trial design, participant selection, interventions, and outcome measures.
- Results: Comprehensive reporting of the trial findings, including participant flow and statistical analyses.
- Discussion: Interpretation of the results, implications for practice, and limitations of the study.
Steps to Complete the CONSORT Checklist of Information to Include When Reporting a Randomized Trial A
Completing the CONSORT Checklist involves several steps:
- Review the checklist thoroughly to understand each item.
- Gather all relevant data and documentation from the trial.
- Draft the report, ensuring each checklist item is addressed.
- Cross-reference the report with the checklist to confirm completeness.
- Seek feedback from colleagues or mentors to enhance clarity and accuracy.
- Finalize the report for submission to a journal or other platform.
Legal Use of the CONSORT Checklist of Information to Include When Reporting a Randomized Trial A
Legally, the CONSORT Checklist is not a binding document but serves as a guideline to enhance the quality of trial reporting. Researchers are encouraged to adhere to the checklist to meet ethical standards in research publication. Compliance with the checklist can help prevent issues related to misrepresentation of data and ensure that the research is conducted and reported responsibly. When submitting research for publication, many journals require adherence to the CONSORT guidelines, emphasizing its importance in the academic and clinical research communities.
Examples of Using the CONSORT Checklist of Information to Include When Reporting a Randomized Trial A
Examples of using the CONSORT Checklist can be found in various published randomized trials. Researchers often cite their adherence to the checklist in their methods section, demonstrating their commitment to transparency. For instance, a trial investigating a new medication may detail participant recruitment, randomization methods, and outcome measures in accordance with the checklist. By providing clear examples of how each element was addressed, researchers can illustrate the robustness of their study and enhance the credibility of their findings.
Quick guide on how to complete consort checklist of information to include when reporting a randomized trial a
Complete CONSORT Checklist Of Information To Include When Reporting A Randomized Trial A seamlessly on any device
Digital document management has become increasingly favored by companies and individuals alike. It offers an ideal environmentally-friendly option to traditional printed and signed documentation, as you can easily locate the necessary form and securely store it online. airSlate SignNow equips you with all the resources required to generate, edit, and electronically sign your documents quickly without interruptions. Manage CONSORT Checklist Of Information To Include When Reporting A Randomized Trial A on any device using airSlate SignNow's Android or iOS applications and enhance any document-centric process today.
How to modify and electronically sign CONSORT Checklist Of Information To Include When Reporting A Randomized Trial A with ease
- Find CONSORT Checklist Of Information To Include When Reporting A Randomized Trial A and click Get Form to begin.
- Utilize the tools we provide to fill out your document.
- Highlight pertinent sections of your documents or obscure sensitive information with features that airSlate SignNow offers specifically for that purpose.
- Create your signature using the Sign tool, which takes mere seconds and holds the same legal validity as a conventional wet ink signature.
- Verify all the details and click on the Done button to save your modifications.
- Choose your preferred method to send your form, whether by email, SMS, invitation link, or download it to your computer.
Say goodbye to lost or misplaced paperwork, tedious form searching, or mistakes that necessitate printing new document copies. airSlate SignNow meets your document management needs in just a few clicks from any device of your choosing. Edit and electronically sign CONSORT Checklist Of Information To Include When Reporting A Randomized Trial A and ensure effective communication at every stage of your form preparation journey with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the consort checklist of information to include when reporting a randomized trial a
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is the CONSORT Checklist Of Information To Include When Reporting A Randomized Trial A?
The CONSORT Checklist Of Information To Include When Reporting A Randomized Trial A is a guideline designed to improve the transparency and completeness of reports of randomized trials. It helps researchers ensure that all essential information is included in their reports, promoting better understanding and replicability of studies.
-
How does airSlate SignNow support the use of the CONSORT Checklist?
airSlate SignNow allows researchers to easily eSign and manage documents related to their randomized trials, ensuring compliance with the CONSORT Checklist Of Information To Include When Reporting A Randomized Trial A. With our platform, users can streamline the documentation process, making it simple to include all necessary information.
-
What are the pricing options for airSlate SignNow?
airSlate SignNow offers several pricing tiers to accommodate different business needs. Each plan is designed to provide access to features that support the documentation required by the CONSORT Checklist Of Information To Include When Reporting A Randomized Trial A, ensuring that users can choose the best fit for their budget.
-
What features does airSlate SignNow provide for project management?
airSlate SignNow includes robust features such as customizable templates, comprehensive tracking, and automated reminders, all of which facilitate project management. These features help users adhere to the CONSORT Checklist Of Information To Include When Reporting A Randomized Trial A by making sure all essential documents are accounted for and properly managed.
-
How can I integrate airSlate SignNow with other tools for my research team?
airSlate SignNow seamlessly integrates with various productivity tools and applications, enhancing collaboration among your research team. This integration ensures that all information required by the CONSORT Checklist Of Information To Include When Reporting A Randomized Trial A is easily accessible and can be shared across platforms, improving workflow efficiency.
-
What are the benefits of using airSlate SignNow in clinical trials?
Using airSlate SignNow streamlines the eSigning and documentation process in clinical trials, helping researchers meet the standards set by the CONSORT Checklist Of Information To Include When Reporting A Randomized Trial A. The platform's user-friendly interface and robust features provide signNow time savings and improve accuracy in trial reporting.
-
Is airSlate SignNow compliant with industry regulations?
Yes, airSlate SignNow is designed to comply with industry regulations, making it a reliable choice for researchers. This compliance ensures that the platform supports the accurate reporting of randomized trials, in line with the CONSORT Checklist Of Information To Include When Reporting A Randomized Trial A.
Get more for CONSORT Checklist Of Information To Include When Reporting A Randomized Trial A
Find out other CONSORT Checklist Of Information To Include When Reporting A Randomized Trial A
- Help Me With eSignature Michigan High Tech Emergency Contact Form
- eSignature Louisiana Insurance Rental Application Later
- eSignature Maryland Insurance Contract Safe
- eSignature Massachusetts Insurance Lease Termination Letter Free
- eSignature Nebraska High Tech Rental Application Now
- How Do I eSignature Mississippi Insurance Separation Agreement
- Help Me With eSignature Missouri Insurance Profit And Loss Statement
- eSignature New Hampshire High Tech Lease Agreement Template Mobile
- eSignature Montana Insurance Lease Agreement Template Online
- eSignature New Hampshire High Tech Lease Agreement Template Free
- How To eSignature Montana Insurance Emergency Contact Form
- eSignature New Jersey High Tech Executive Summary Template Free
- eSignature Oklahoma Insurance Warranty Deed Safe
- eSignature Pennsylvania High Tech Bill Of Lading Safe
- eSignature Washington Insurance Work Order Fast
- eSignature Utah High Tech Warranty Deed Free
- How Do I eSignature Utah High Tech Warranty Deed
- eSignature Arkansas Legal Affidavit Of Heirship Fast
- Help Me With eSignature Colorado Legal Cease And Desist Letter
- How To eSignature Connecticut Legal LLC Operating Agreement