MDSAP QMS F00041001 Risk Management Process Flowchart Fda Form


What is the MDSAP QMS F00041001 Risk Management Process Flowchart FDA?
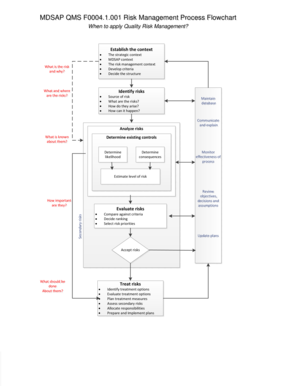
The MDSAP QMS F00041001 Risk Management Process Flowchart FDA is a structured document designed to guide organizations in implementing risk management practices compliant with the Medical Device Single Audit Program (MDSAP). This flowchart outlines the steps necessary for identifying, assessing, and mitigating risks associated with medical devices. It serves as a visual representation of the risk management process, ensuring that all critical elements are addressed systematically. Understanding this flowchart is essential for manufacturers aiming to meet regulatory requirements and enhance product safety.
How to Use the MDSAP QMS F00041001 Risk Management Process Flowchart FDA
Using the MDSAP QMS F00041001 Risk Management Process Flowchart FDA involves several key steps. First, familiarize yourself with the flowchart's structure and the specific stages it outlines. Begin by identifying potential hazards related to your medical device. Next, assess the risks associated with these hazards, considering both the likelihood of occurrence and the severity of potential harm. The flowchart will guide you through the process of evaluating these risks and determining appropriate mitigation strategies. Finally, document your findings and decisions at each stage to ensure compliance with regulatory standards.
Key Elements of the MDSAP QMS F00041001 Risk Management Process Flowchart FDA
The MDSAP QMS F00041001 Risk Management Process Flowchart FDA includes several key elements crucial for effective risk management. These elements typically encompass:
- Risk Identification: Recognizing potential hazards associated with the medical device.
- Risk Analysis: Evaluating the identified risks in terms of likelihood and impact.
- Risk Evaluation: Comparing estimated risks against predefined acceptance criteria.
- Risk Control: Implementing measures to mitigate identified risks.
- Monitoring and Review: Continuously assessing the effectiveness of risk controls and making necessary adjustments.
These elements ensure a comprehensive approach to risk management, aligning with FDA regulations and enhancing product safety.
Steps to Complete the MDSAP QMS F00041001 Risk Management Process Flowchart FDA
Completing the MDSAP QMS F00041001 Risk Management Process Flowchart FDA involves a systematic approach. Follow these steps:
- Gather Information: Collect relevant data about the medical device, including design specifications and user feedback.
- Identify Risks: Use brainstorming sessions and historical data to identify potential hazards.
- Analyze Risks: Assess each risk for its likelihood of occurrence and potential impact on patient safety.
- Evaluate Risks: Determine whether the identified risks are acceptable based on established criteria.
- Implement Controls: Develop and apply strategies to mitigate unacceptable risks.
- Document Findings: Record all steps taken, decisions made, and outcomes achieved for compliance and future reference.
By following these steps, organizations can effectively manage risks associated with their medical devices.
Legal Use of the MDSAP QMS F00041001 Risk Management Process Flowchart FDA
The legal use of the MDSAP QMS F00041001 Risk Management Process Flowchart FDA is governed by regulations set forth by the FDA and other relevant authorities. Compliance with these regulations is essential for ensuring that the risk management process meets legal standards. Organizations must ensure that their use of the flowchart aligns with the guidelines established under the MDSAP framework, which emphasizes the importance of thorough documentation and adherence to risk management principles. Failure to comply with these legal requirements can result in penalties and hinder market access for medical devices.
Quick guide on how to complete mdsap qms f00041001 risk management process flowchart fda
Prepare MDSAP QMS F00041001 Risk Management Process Flowchart Fda effortlessly on any device
Online document management has become favored by businesses and individuals alike. It offers a superb eco-friendly substitute for traditional printed and signed documents, as you can access the correct template and safely store it online. airSlate SignNow equips you with all the resources necessary to create, modify, and electronically sign your documents quickly without delays. Manage MDSAP QMS F00041001 Risk Management Process Flowchart Fda on any device with airSlate SignNow’s Android or iOS applications and enhance any document-based process today.
How to modify and eSign MDSAP QMS F00041001 Risk Management Process Flowchart Fda without any hassle
- Obtain MDSAP QMS F00041001 Risk Management Process Flowchart Fda and then click Get Form to begin.
- Utilize the tools we provide to complete your form.
- Emphasize important sections of your documents or redact sensitive information with tools that airSlate SignNow offers specifically for that purpose.
- Create your signature using the Sign feature, which takes seconds and holds the same legal validity as a conventional wet ink signature.
- Review the information and then click on the Done button to preserve your changes.
- Choose your preferred method to send your form, via email, SMS, or invite link, or download it to your computer.
Say goodbye to lost or misplaced files, tedious form searching, or errors that necessitate printing new document copies. airSlate SignNow fulfills all your document management requirements in just a few clicks from any device you choose. Modify and eSign MDSAP QMS F00041001 Risk Management Process Flowchart Fda and ensure excellent communication at every stage of the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the mdsap qms f00041001 risk management process flowchart fda
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is the MDSAP QMS F00041001 Risk Management Process Flowchart Fda?
The MDSAP QMS F00041001 Risk Management Process Flowchart Fda is a structured visualization of the risk management processes mandated by the FDA under the Medical Device Single Audit Program. It aids organizations in complying with applicable regulations and maintaining quality management standards. This flowchart simplifies the understanding and implementation of risk management procedures essential for medical device manufacturers.
-
How can airSlate SignNow help in the MDSAP QMS F00041001 process?
airSlate SignNow offers an efficient eSigning solution that can streamline the documentation process involved in the MDSAP QMS F00041001 Risk Management Process Flowchart Fda. Our platform allows teams to easily send, sign, and manage critical documents, ensuring compliance and enhancing collaboration. This simplifies the workflow and ensures that all processes align with regulatory requirements.
-
What are the pricing options for airSlate SignNow?
AirSlate SignNow provides flexible pricing plans tailored to various business needs, including those requiring compliance with the MDSAP QMS F00041001 Risk Management Process Flowchart Fda. Plans vary based on features, number of users, and document volume. You can start with a free trial to explore how our solutions can meet your requirements.
-
Is airSlate SignNow compliant with FDA regulations?
Yes, airSlate SignNow is designed with compliance in mind, making it a suitable choice for those adhering to the MDSAP QMS F00041001 Risk Management Process Flowchart Fda. Our document handling processes ensure that all signatures and approvals meet regulatory standards. This compliance is crucial for businesses in the medical device field.
-
What are the key features of airSlate SignNow relevant to MDSAP QMS F00041001?
AirSlate SignNow includes features such as document templates, real-time tracking, and secure eSignature capabilities, all vital for the MDSAP QMS F00041001 Risk Management Process Flowchart Fda. These features help improve efficiency, reduce errors, and maintain compliance with regulatory standards. Additionally, our platform can integrate with existing systems for seamless operations.
-
How does airSlate SignNow ensure document security?
AirSlate SignNow prioritizes security, employing encryption and secure storage protocols to protect documents related to the MDSAP QMS F00041001 Risk Management Process Flowchart Fda. Access controls and authentication measures enhance security, providing peace of mind that sensitive information remains confidential. We ensure your documents are safe at every step of the signing process.
-
Can airSlate SignNow integrate with other software?
Absolutely! AirSlate SignNow integrates easily with various software tools, providing a seamless experience while managing the MDSAP QMS F00041001 Risk Management Process Flowchart Fda. Integration with CRM systems, document management platforms, and other tools enhances productivity and ensures that your team's workflow remains uninterrupted. These integrations help in maintaining organized and compliant records.
Get more for MDSAP QMS F00041001 Risk Management Process Flowchart Fda
Find out other MDSAP QMS F00041001 Risk Management Process Flowchart Fda
- Sign Arizona Plumbing Rental Application Secure
- Sign Colorado Plumbing Emergency Contact Form Now
- Sign Colorado Plumbing Emergency Contact Form Free
- How Can I Sign Connecticut Plumbing LLC Operating Agreement
- Sign Illinois Plumbing Business Plan Template Fast
- Sign Plumbing PPT Idaho Free
- How Do I Sign Wyoming Life Sciences Confidentiality Agreement
- Sign Iowa Plumbing Contract Safe
- Sign Iowa Plumbing Quitclaim Deed Computer
- Sign Maine Plumbing LLC Operating Agreement Secure
- How To Sign Maine Plumbing POA
- Sign Maryland Plumbing Letter Of Intent Myself
- Sign Hawaii Orthodontists Claim Free
- Sign Nevada Plumbing Job Offer Easy
- Sign Nevada Plumbing Job Offer Safe
- Sign New Jersey Plumbing Resignation Letter Online
- Sign New York Plumbing Cease And Desist Letter Free
- Sign Alabama Real Estate Quitclaim Deed Free
- How Can I Sign Alabama Real Estate Affidavit Of Heirship
- Can I Sign Arizona Real Estate Confidentiality Agreement