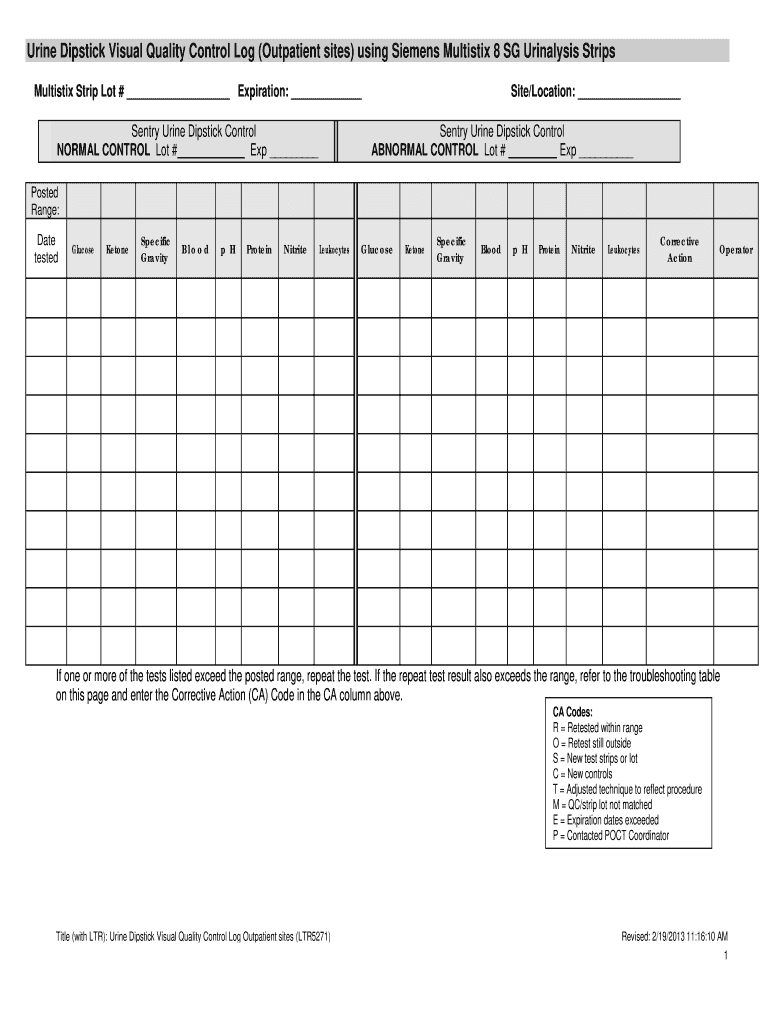
Siemens Multistix 10 Sg Quality Control Log Form


What is the Siemens Multistix 10 Sg Quality Control Log
The Siemens Multistix 10 Sg Quality Control Log is a critical document used in laboratories to ensure the accuracy and reliability of urinalysis results. This log records the quality control tests performed on the Siemens Multistix 10 Sg reagent strips, which are commonly used for urine testing. It helps laboratory personnel track the performance of the testing strips, ensuring that they meet the required standards for clinical use.
Maintaining a quality control log is essential for compliance with regulatory standards and for maintaining the integrity of test results. The log typically includes information such as the date of testing, the control solutions used, the results obtained, and any corrective actions taken if results fall outside acceptable ranges.
How to use the Siemens Multistix 10 Sg Quality Control Log
Using the Siemens Multistix 10 Sg Quality Control Log involves several straightforward steps. First, ensure that you have the appropriate quality control solutions that correspond to the reagent strips being tested. Next, enter the date and time of the quality control test in the log.
After conducting the quality control test, record the results in the log. It is important to compare these results with the expected values provided by the manufacturer. If the results are within the acceptable range, you can proceed with urinalysis testing. However, if results are outside the expected range, document the issue and take corrective actions, which should also be noted in the log.
Steps to complete the Siemens Multistix 10 Sg Quality Control Log
Completing the Siemens Multistix 10 Sg Quality Control Log involves a series of methodical steps:
- Gather all necessary materials, including the quality control solutions and the log.
- Perform the quality control test according to the manufacturer's instructions.
- Record the date and time of the test in the log.
- Document the results of the quality control test, ensuring they are compared against the expected values.
- If results are satisfactory, note this in the log. If not, document any corrective actions taken.
- Store the log securely for future reference and compliance audits.
Key elements of the Siemens Multistix 10 Sg Quality Control Log
Several key elements must be included in the Siemens Multistix 10 Sg Quality Control Log to ensure its effectiveness:
- Date and Time: Record when the quality control test was performed.
- Control Solution Lot Number: Document the lot number of the control solutions used.
- Test Results: Clearly indicate the results of the quality control tests.
- Corrective Actions: Note any actions taken if results were outside the acceptable range.
- Signature of the Tester: Include the name or initials of the person conducting the test for accountability.
Legal use of the Siemens Multistix 10 Sg Quality Control Log
The legal use of the Siemens Multistix 10 Sg Quality Control Log is essential for compliance with health regulations and standards set by governing bodies. This log serves as a formal record that can be reviewed during audits or inspections. Proper documentation is vital for demonstrating adherence to quality control protocols, which can impact laboratory accreditation and liability in cases of erroneous test results.
It is important to maintain the log accurately and securely, as discrepancies or incomplete records may lead to legal repercussions for the laboratory. Regular training for staff on the importance of maintaining the quality control log can help ensure compliance and uphold the integrity of laboratory operations.
Quick guide on how to complete urine dipstick visual quality control log outpatient massgeneral
Prepare Siemens Multistix 10 Sg Quality Control Log seamlessly on any device
Digital document management has become increasingly sought after by businesses and individuals alike. It offers an ideal eco-friendly substitute for traditional printed and signed documents, as you can easily access the right form and securely save it online. airSlate SignNow equips you with all the necessary tools to create, modify, and eSign your documents promptly without interruptions. Handle Siemens Multistix 10 Sg Quality Control Log on any platform with airSlate SignNow Android or iOS applications and enhance any document-related process today.
How to modify and eSign Siemens Multistix 10 Sg Quality Control Log effortlessly
- Find Siemens Multistix 10 Sg Quality Control Log and click on Get Form to begin.
- Make use of the tools we offer to fill out your form.
- Mark essential sections of your documents or obscure sensitive details with tools specifically designed by airSlate SignNow for that purpose.
- Create your signature using the Sign tool, which only takes a few seconds and carries the same legal validity as a conventional wet ink signature.
- Review all the details and click on the Done button to save your changes.
- Select your preferred method for sending your form, whether by email, text message (SMS), or an invitation link, or download it to your computer.
Forget about lost or mislaid documents, tedious form searching, or errors that necessitate printing new copies. airSlate SignNow addresses your document management needs in just a few clicks from any device you prefer. Modify and eSign Siemens Multistix 10 Sg Quality Control Log and guarantee excellent communication at any stage of your form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the urine dipstick visual quality control log outpatient massgeneral
How to create an electronic signature for the Urine Dipstick Visual Quality Control Log Outpatient Massgeneral in the online mode
How to generate an electronic signature for the Urine Dipstick Visual Quality Control Log Outpatient Massgeneral in Chrome
How to make an electronic signature for putting it on the Urine Dipstick Visual Quality Control Log Outpatient Massgeneral in Gmail
How to make an eSignature for the Urine Dipstick Visual Quality Control Log Outpatient Massgeneral straight from your smart phone
How to make an electronic signature for the Urine Dipstick Visual Quality Control Log Outpatient Massgeneral on iOS
How to generate an eSignature for the Urine Dipstick Visual Quality Control Log Outpatient Massgeneral on Android
People also ask
-
What is a urinalysis quality control log?
A urinalysis quality control log is a comprehensive record that tracks the performance and accuracy of urinalysis testing processes. It helps laboratories maintain high standards and compliance by documenting routine quality controls, ensuring reliable test results.
-
How can airSlate SignNow improve my urinalysis quality control log management?
airSlate SignNow provides an efficient platform for creating, managing, and signing your urinalysis quality control log. With its user-friendly interface, you can easily share logs with team members, ensuring that everyone has the most up-to-date information. This enhances collaboration and streamlines compliance tracking.
-
Is airSlate SignNow cost-effective for managing urinalysis quality control logs?
Yes, airSlate SignNow offers a cost-effective solution for managing urinalysis quality control logs. Our pricing plans are designed to fit the needs of businesses of all sizes, ensuring you receive great value for an essential management tool while maintaining budget efficiency.
-
What features does airSlate SignNow offer for maintaining a urinalysis quality control log?
airSlate SignNow includes features such as customizable templates for your urinalysis quality control log, automated reminders for regular updates, and easy eSigning options. These features simplify the process of maintaining an accurate and timely log, ultimately improving lab efficiency.
-
Can I integrate airSlate SignNow with other lab management tools for my urinalysis quality control log?
Absolutely! airSlate SignNow can be seamlessly integrated with various lab management tools to enhance the utility of your urinalysis quality control log. This allows for smoother data transfer and better overall management of laboratory processes.
-
What are the benefits of digitizing my urinalysis quality control log?
Digitizing your urinalysis quality control log with airSlate SignNow increases accessibility and promotes data accuracy. It allows for quick updates and easy retrieval of information, ensuring your quality control measures are always up to date and compliant with regulatory standards.
-
How does airSlate SignNow ensure the security of my urinalysis quality control log?
airSlate SignNow prioritizes security and compliance by implementing advanced encryption and secure storage protocols. Your urinalysis quality control log is protected, ensuring that sensitive data remains confidential and compliant with industry regulations.
Get more for Siemens Multistix 10 Sg Quality Control Log
Find out other Siemens Multistix 10 Sg Quality Control Log
- How Can I Electronic signature Tennessee Legal Warranty Deed
- Electronic signature Texas Legal Lease Agreement Template Free
- Can I Electronic signature Texas Legal Lease Agreement Template
- How To Electronic signature Texas Legal Stock Certificate
- How Can I Electronic signature Texas Legal POA
- Electronic signature West Virginia Orthodontists Living Will Online
- Electronic signature Legal PDF Vermont Online
- How Can I Electronic signature Utah Legal Separation Agreement
- Electronic signature Arizona Plumbing Rental Lease Agreement Myself
- Electronic signature Alabama Real Estate Quitclaim Deed Free
- Electronic signature Alabama Real Estate Quitclaim Deed Safe
- Electronic signature Colorado Plumbing Business Plan Template Secure
- Electronic signature Alaska Real Estate Lease Agreement Template Now
- Electronic signature Colorado Plumbing LLC Operating Agreement Simple
- Electronic signature Arizona Real Estate Business Plan Template Free
- Electronic signature Washington Legal Contract Safe
- How To Electronic signature Arkansas Real Estate Contract
- Electronic signature Idaho Plumbing Claim Myself
- Electronic signature Kansas Plumbing Business Plan Template Secure
- Electronic signature Louisiana Plumbing Purchase Order Template Simple