Sample Clinical Quality Management Regulatory File Review Tool Sample Clinical Quality Management Regulatory File Review Tool Ni Form


Understanding the Sample Clinical Quality Management Regulatory File Review Tool
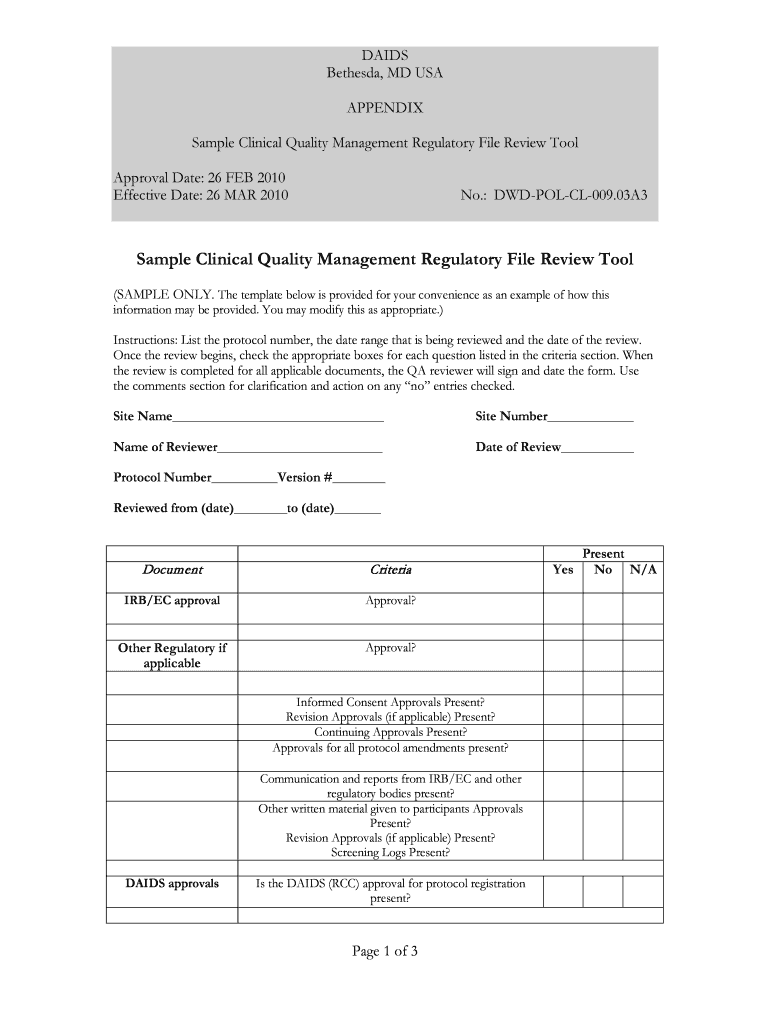
The Sample Clinical Quality Management Regulatory File Review Tool is designed to assist organizations in ensuring compliance with clinical quality standards set forth by regulatory bodies such as the National Institute of Allergy and Infectious Diseases (NIAID) and the National Institutes of Health (NIH). This tool helps in systematically reviewing clinical quality management files, ensuring that all necessary documentation is present and adheres to regulatory requirements. It serves as a comprehensive checklist that organizations can utilize to maintain high standards in clinical research and management.
How to Effectively Use the Sample Clinical Quality Management Regulatory File Review Tool
To utilize the Sample Clinical Quality Management Regulatory File Review Tool effectively, begin by familiarizing yourself with its structure and the specific criteria outlined within. Each section of the tool corresponds to different regulatory requirements. Users should methodically go through each item, ensuring that all necessary documents are included and that they meet the specified standards. Regularly updating the tool based on the latest regulatory changes is also crucial to maintaining compliance.
Obtaining the Sample Clinical Quality Management Regulatory File Review Tool
The Sample Clinical Quality Management Regulatory File Review Tool can typically be obtained through official channels associated with NIAID and NIH. Organizations may access the tool via their respective websites, where they often provide downloadable resources. It is advisable to check for the most recent version to ensure compliance with current regulations. Additionally, professional networks and clinical research organizations may offer access to this tool as part of their resources.
Key Elements of the Sample Clinical Quality Management Regulatory File Review Tool
This tool includes several key elements that are essential for effective regulatory file review. These elements encompass documentation requirements, quality control measures, and compliance checklists specific to clinical quality management. Each element is designed to guide users through the review process, ensuring that all necessary aspects of regulatory compliance are covered. Understanding these elements is vital for successful implementation and adherence to clinical standards.
Legal Considerations for Using the Sample Clinical Quality Management Regulatory File Review Tool
When using the Sample Clinical Quality Management Regulatory File Review Tool, it is important to consider the legal implications of compliance with federal regulations. Organizations should ensure that their use of the tool aligns with the legal requirements set forth by regulatory agencies. This includes maintaining accurate records, ensuring data privacy, and adhering to ethical standards in clinical research. Non-compliance can lead to legal repercussions, making it essential to use the tool correctly.
Steps to Complete the Sample Clinical Quality Management Regulatory File Review Tool
Completing the Sample Clinical Quality Management Regulatory File Review Tool involves several systematic steps. First, gather all relevant documentation required for review. Next, use the tool to cross-reference each document against the checklist provided. Ensure that all items are complete and compliant with regulatory standards. After completing the review, document any findings and take corrective actions if necessary. Finally, keep a record of the completed review for future reference and audits.
Quick guide on how to complete sample clinical quality management regulatory file review tool sample clinical quality management regulatory file review tool
Effortlessly Prepare [SKS] on Any Device
The management of online documents has become increasingly favored by businesses and individuals alike. It serves as an ideal eco-friendly alternative to conventional printed and signed documents, allowing you to find the right template and securely store it online. airSlate SignNow equips you with all the tools necessary to create, modify, and electronically sign your documents swiftly without delays. Manage [SKS] on any device with airSlate SignNow’s Android or iOS applications and simplify any document-related process immediately.
How to Modify and eSign [SKS] with Ease
- Obtain [SKS] and click Get Form to begin.
- Utilize the tools we provide to fill out your form.
- Emphasize pertinent sections of your documents or obscure sensitive details with tools specifically designed for that purpose by airSlate SignNow.
- Generate your signature using the Sign feature, which takes moments and carries the same legal validity as a conventional wet ink signature.
- Carefully review the information and click on the Done button to save your updates.
- Select how you prefer to submit your form, either via email, SMS, or invite link, or download it to your computer.
Eliminate concerns about lost or misplaced documents, tedious form searches, or mistakes that necessitate printing new copies. airSlate SignNow meets your document management needs in just a few clicks from any device you choose. Edit and eSign [SKS] and guarantee effective communication throughout your form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Related searches to Sample Clinical Quality Management Regulatory File Review Tool Sample Clinical Quality Management Regulatory File Review Tool Ni
Create this form in 5 minutes!
How to create an eSignature for the sample clinical quality management regulatory file review tool sample clinical quality management regulatory file review tool
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is the Sample Clinical Quality Management Regulatory File Review Tool Niaid Nih?
The Sample Clinical Quality Management Regulatory File Review Tool Niaid Nih is an advanced solution designed for managing and reviewing clinical quality files. It ensures compliance with regulatory requirements, enhancing the quality of clinical trials through organized document management. By using this tool, you can streamline your review processes and maintain high standards in clinical research.
-
How does the Sample Clinical Quality Management Regulatory File Review Tool benefit my organization?
Utilizing the Sample Clinical Quality Management Regulatory File Review Tool Niaid Nih allows organizations to improve efficiency and accuracy in clinical file reviews. The tool is designed to minimize errors and reduce the time spent on regulatory compliance tasks. This leads to more focused research efforts and better resource allocation.
-
What features are included in the Sample Clinical Quality Management Regulatory File Review Tool?
The Sample Clinical Quality Management Regulatory File Review Tool Niaid Nih offers features such as document version control, automated workflows, and real-time collaboration tools. These features help teams manage file revisions and ensure all stakeholders are aligned. Additionally, it supports various formats to enhance accessibility.
-
Is the Sample Clinical Quality Management Regulatory File Review Tool customizable?
Yes, the Sample Clinical Quality Management Regulatory File Review Tool Niaid Nih can be customized to meet the specific needs of your organization. You can tailor workflows, document templates, and user permissions to match your operational processes. This flexibility ensures that the tool adapts seamlessly to your existing system.
-
What is the pricing model for the Sample Clinical Quality Management Regulatory File Review Tool?
The pricing for the Sample Clinical Quality Management Regulatory File Review Tool Niaid Nih varies based on the size of your organization and the number of users. airSlate SignNow offers competitive pricing plans that provide signNow value for the features included. For a detailed quote, it's best to contact our sales team for personalized assistance.
-
Can I integrate the Sample Clinical Quality Management Regulatory File Review Tool with other software?
Absolutely! The Sample Clinical Quality Management Regulatory File Review Tool Niaid Nih is designed to be easily integrated with various software applications. This includes popular document management systems and CRM tools, facilitating a seamless workflow across your digital environment.
-
How secure is the Sample Clinical Quality Management Regulatory File Review Tool?
Security is a top priority with the Sample Clinical Quality Management Regulatory File Review Tool Niaid Nih. The tool employs advanced encryption methods and complies with industry standards to safeguard your documents and data. Users benefit from secure access controls and audit trails to enhance accountability.
Get more for Sample Clinical Quality Management Regulatory File Review Tool Sample Clinical Quality Management Regulatory File Review Tool Ni
Find out other Sample Clinical Quality Management Regulatory File Review Tool Sample Clinical Quality Management Regulatory File Review Tool Ni
- eSignature Arkansas Non-Compete Agreement Later
- Can I eSignature Arizona Non-Compete Agreement
- How Do I eSignature New Jersey Non-Compete Agreement
- eSignature Tennessee Non-Compete Agreement Myself
- How To eSignature Colorado LLC Operating Agreement
- Help Me With eSignature North Carolina LLC Operating Agreement
- eSignature Oregon LLC Operating Agreement Online
- eSignature Wyoming LLC Operating Agreement Online
- eSignature Wyoming LLC Operating Agreement Computer
- eSignature Wyoming LLC Operating Agreement Later
- eSignature Wyoming LLC Operating Agreement Free
- How To eSignature Wyoming LLC Operating Agreement
- eSignature California Commercial Lease Agreement Template Myself
- eSignature California Commercial Lease Agreement Template Easy
- eSignature Florida Commercial Lease Agreement Template Easy
- eSignature Texas Roommate Contract Easy
- eSignature Arizona Sublease Agreement Template Free
- eSignature Georgia Sublease Agreement Template Online
- eSignature Arkansas Roommate Rental Agreement Template Mobile
- eSignature Maryland Roommate Rental Agreement Template Free