Application for Approval of Research Project Involving Human Subjects Form


What is the Application For Approval Of Research Project Involving Human Subjects
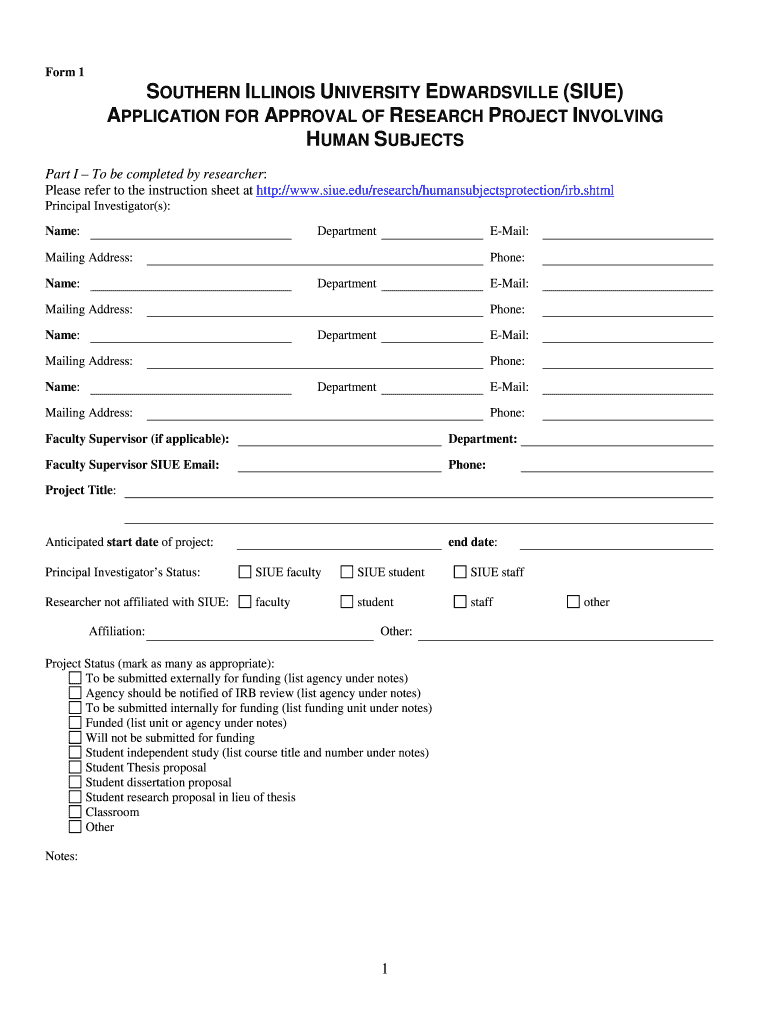
The Application For Approval Of Research Project Involving Human Subjects is a formal document required by institutions and regulatory bodies to ensure that research projects involving human participants are ethically sound and comply with federal regulations. This application outlines the purpose of the research, the methods to be used, and the measures taken to protect the rights and welfare of participants. It is essential for researchers to obtain approval before commencing any project that involves human subjects to ensure compliance with ethical standards and legal requirements.
Steps to complete the Application For Approval Of Research Project Involving Human Subjects
Completing the Application For Approval Of Research Project Involving Human Subjects involves several key steps. First, researchers should gather all necessary information about the project, including objectives, methodologies, and participant recruitment strategies. Next, they must outline the informed consent process, detailing how participants will be informed about the study and their rights. After drafting the application, researchers should review it for completeness and clarity. Finally, submit the application to the appropriate institutional review board (IRB) or ethics committee for evaluation.
Key elements of the Application For Approval Of Research Project Involving Human Subjects
The application includes several critical components that must be addressed. Key elements typically consist of:
- Research Objectives: Clearly defined goals and hypotheses of the study.
- Methodology: Detailed description of research methods, including data collection and analysis.
- Participant Information: Criteria for inclusion and exclusion, as well as recruitment strategies.
- Informed Consent: Explanation of how participants will be informed and how consent will be obtained.
- Risk Assessment: Identification of potential risks to participants and measures to mitigate them.
Legal use of the Application For Approval Of Research Project Involving Human Subjects
The legal use of this application is governed by federal regulations, including the Common Rule, which outlines the ethical principles for research involving human subjects. Compliance with these regulations is essential to protect participants and ensure the integrity of the research. Researchers must adhere to institutional policies and guidelines, which may vary by institution, and ensure that all necessary approvals are obtained before initiating the research.
Form Submission Methods
Researchers can submit the Application For Approval Of Research Project Involving Human Subjects through various methods, depending on the institution's requirements. Common submission methods include:
- Online Submission: Many institutions offer electronic submission portals for ease of access and tracking.
- Mail: Physical submission of printed applications may be required by some institutions.
- In-Person Submission: Researchers may need to deliver the application directly to the IRB or ethics committee office.
Eligibility Criteria
Eligibility to submit the Application For Approval Of Research Project Involving Human Subjects typically requires the researcher to be affiliated with an institution that has an IRB. Additionally, researchers must demonstrate that their project meets ethical standards and complies with federal regulations. This includes providing adequate training in human subjects research and ensuring that all team members involved in the study are aware of their responsibilities regarding participant welfare.
Quick guide on how to complete application for approval of research project involving human subjects
Effortlessly prepare [SKS] on any gadget
Digital document management has gained signNow traction among organizations and individuals alike. It offers an excellent eco-friendly substitute for traditional printed and signed documents, as you can easily access the correct form and securely store it online. airSlate SignNow provides you with all the resources necessary to create, modify, and eSign your documents quickly without interruptions. Manage [SKS] on any gadget through airSlate SignNow's Android or iOS applications and simplify any document-related process right away.
Steps to modify and eSign [SKS] with ease
- Find [SKS] and click Get Form to begin.
- Utilize the tools we provide to fill out your form.
- Emphasize important sections of your documents or redact sensitive information using the tools provided by airSlate SignNow specifically for that purpose.
- Create your eSignature with the Sign tool, which takes just seconds and holds the same legal authority as a conventional wet ink signature.
- Review the details and click on the Done button to save your modifications.
- Select how you wish to share your form, whether by email, SMS, or invitation link, or download it to your computer.
Say goodbye to lost or misplaced documents, tedious form searching, or mistakes that necessitate printing new copies. airSlate SignNow fulfills all your document management needs in just a few clicks from your chosen device. Modify and eSign [SKS] and ensure excellent communication throughout the form preparation stages with airSlate SignNow.
Create this form in 5 minutes or less
Related searches to Application For Approval Of Research Project Involving Human Subjects
Create this form in 5 minutes!
How to create an eSignature for the application for approval of research project involving human subjects
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is the Application For Approval Of Research Project Involving Human Subjects?
The Application For Approval Of Research Project Involving Human Subjects is a formal request submitted to an ethics committee or institutional review board. It outlines the research project, detailing how human subjects will be involved and ensuring their rights and welfare are protected. This application is crucial for compliance with ethical standards in research.
-
How can airSlate SignNow assist with the Application For Approval Of Research Project Involving Human Subjects?
airSlate SignNow provides a streamlined platform for managing the Application For Approval Of Research Project Involving Human Subjects. With its eSignature capabilities, researchers can easily obtain necessary approvals from stakeholders and ethics committees. This simplifies the process, ensuring that all documentation is securely signed and stored.
-
What are the pricing options for using airSlate SignNow for research applications?
airSlate SignNow offers various pricing plans to accommodate different needs, including options for individuals and teams. Each plan provides access to features that can enhance the Application For Approval Of Research Project Involving Human Subjects process. You can choose a plan that fits your budget while ensuring compliance and efficiency.
-
What features does airSlate SignNow offer for managing research project applications?
airSlate SignNow includes features such as customizable templates, automated workflows, and secure eSigning. These tools are particularly beneficial for the Application For Approval Of Research Project Involving Human Subjects, allowing researchers to create, send, and track applications effortlessly. This enhances collaboration and speeds up the approval process.
-
Are there any integrations available with airSlate SignNow for research applications?
Yes, airSlate SignNow integrates seamlessly with various applications and platforms, enhancing its functionality for the Application For Approval Of Research Project Involving Human Subjects. You can connect it with tools like Google Drive, Dropbox, and CRM systems to streamline document management and collaboration. This ensures a cohesive workflow across your research projects.
-
What are the benefits of using airSlate SignNow for research project approvals?
Using airSlate SignNow for the Application For Approval Of Research Project Involving Human Subjects offers numerous benefits, including increased efficiency and reduced turnaround times. The platform's user-friendly interface makes it easy for researchers to manage documents and obtain necessary signatures. Additionally, it enhances compliance with regulatory requirements.
-
Is airSlate SignNow secure for handling sensitive research applications?
Absolutely, airSlate SignNow prioritizes security, ensuring that all documents related to the Application For Approval Of Research Project Involving Human Subjects are protected. The platform employs advanced encryption and complies with industry standards to safeguard sensitive information. Researchers can trust that their data is secure throughout the approval process.
Get more for Application For Approval Of Research Project Involving Human Subjects
Find out other Application For Approval Of Research Project Involving Human Subjects
- How Do I Electronic signature Nevada Car Dealer PDF
- How To Electronic signature South Carolina Banking Document
- Can I Electronic signature New York Car Dealer Document
- How To Electronic signature North Carolina Car Dealer Word
- How Do I Electronic signature North Carolina Car Dealer Document
- Can I Electronic signature Ohio Car Dealer PPT
- How Can I Electronic signature Texas Banking Form
- How Do I Electronic signature Pennsylvania Car Dealer Document
- How To Electronic signature South Carolina Car Dealer Document
- Can I Electronic signature South Carolina Car Dealer Document
- How Can I Electronic signature Texas Car Dealer Document
- How Do I Electronic signature West Virginia Banking Document
- How To Electronic signature Washington Car Dealer Document
- Can I Electronic signature West Virginia Car Dealer Document
- How Do I Electronic signature West Virginia Car Dealer Form
- How Can I Electronic signature Wisconsin Car Dealer PDF
- How Can I Electronic signature Wisconsin Car Dealer Form
- How Do I Electronic signature Montana Business Operations Presentation
- How To Electronic signature Alabama Charity Form
- How To Electronic signature Arkansas Construction Word