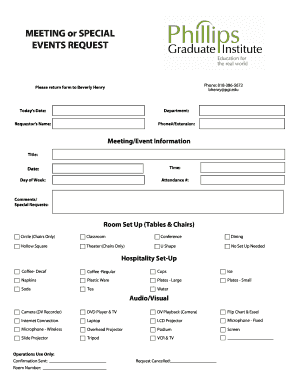
Teleconference, to Discuss in Depth the Draft Revised Plan and Form


Understanding the Teleconference for Discussing the Draft Revised Plan
The teleconference to discuss the draft revised plan serves as a platform for stakeholders to engage in comprehensive dialogue regarding proposed changes. This format allows participants to share insights, raise concerns, and contribute to the decision-making process. Typically, the teleconference is organized by a governing body or organization responsible for the draft plan. It is essential for all involved parties to prepare adequately by reviewing the draft in advance to facilitate informed discussions.
How to Participate in the Teleconference
To take part in the teleconference, participants usually need to register in advance. Registration details are often provided through official announcements or organizational websites. Once registered, attendees receive a link or dial-in information to access the teleconference. It is advisable to join the session a few minutes early to ensure a smooth connection and to address any technical issues that may arise.
Key Components of the Teleconference
During the teleconference, several key components are typically addressed, including:
- Overview of the draft revised plan: A summary of the main changes and objectives outlined in the draft.
- Stakeholder feedback: An opportunity for participants to voice their opinions and suggestions regarding the proposed revisions.
- Next steps: A discussion on the timeline for finalizing the plan and any additional meetings or actions required.
Legal Considerations for the Teleconference
It is important to ensure that the teleconference complies with relevant legal requirements. This may include adherence to open meeting laws, which mandate that certain discussions be accessible to the public. Additionally, any materials shared during the teleconference should be available for public review, ensuring transparency in the decision-making process.
Examples of Teleconference Usage
Teleconferences are commonly used in various contexts, such as:
- Government agencies: To discuss policy changes or draft regulations.
- Non-profit organizations: For community engagement and feedback on proposed initiatives.
- Corporate settings: To gather input from employees or stakeholders on strategic plans.
Steps for Effective Teleconference Engagement
To maximize the effectiveness of the teleconference, participants can follow these steps:
- Prepare: Review the draft revised plan thoroughly before the meeting.
- Listen actively: Pay attention to the discussions and take notes on key points.
- Contribute thoughtfully: Share insights and questions that can help clarify the draft's implications.
- Follow up: Engage with additional resources or meetings as necessary to stay informed on the plan's progress.
Quick guide on how to complete teleconference to discuss in depth the draft revised plan and
Complete [SKS] effortlessly on any device
Digital document management has become increasingly popular among businesses and individuals. It offers an ideal environmentally friendly alternative to conventional printed and signed documents, as you can easily access the appropriate form and securely store it online. airSlate SignNow equips you with all the necessary tools to create, modify, and electronically sign your documents swiftly without delays. Handle [SKS] on any device using airSlate SignNow's Android or iOS applications and enhance any document-centric task today.
How to modify and electronically sign [SKS] with ease
- Obtain [SKS] and click Get Form to begin.
- Utilize the tools we offer to fill out your form.
- Emphasize relevant sections of the documents or redact sensitive information with tools that airSlate SignNow specifically provides for that purpose.
- Create your signature using the Sign tool, which takes seconds and carries the same legal validity as a traditional wet ink signature.
- Review the information and click on the Done button to save your modifications.
- Choose how you wish to send your form, via email, text message (SMS), or invitation link, or download it to your computer.
Eliminate concerns about lost or misplaced files, cumbersome form searching, or errors that necessitate printing new document copies. airSlate SignNow fulfills all your document management needs in just a few clicks from any device of your choosing. Adjust and electronically sign [SKS] and ensure excellent communication at any point in the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Related searches to Teleconference, To Discuss In depth The Draft Revised Plan And
Create this form in 5 minutes!
How to create an eSignature for the teleconference to discuss in depth the draft revised plan and
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is the purpose of a teleconference to discuss in depth the draft revised plan?
A teleconference to discuss in depth the draft revised plan allows stakeholders to collaborate effectively, ensuring that all voices are heard. This format facilitates real-time communication, enabling participants to clarify points and make informed decisions. Utilizing airSlate SignNow during these discussions can streamline document sharing and signing.
-
How can airSlate SignNow enhance my teleconference experience?
airSlate SignNow enhances your teleconference experience by providing a seamless way to share and eSign documents during discussions. Participants can review and sign documents in real-time, which helps to expedite the decision-making process. This integration ensures that all necessary paperwork is completed efficiently while you focus on the draft revised plan.
-
What features does airSlate SignNow offer for teleconferences?
airSlate SignNow offers features such as document sharing, eSigning, and real-time collaboration tools that are perfect for teleconferences. These features allow participants to engage with the draft revised plan directly, making it easier to address any concerns or suggestions. The platform is designed to keep your discussions organized and productive.
-
Is there a cost associated with using airSlate SignNow for teleconferences?
Yes, there is a cost associated with using airSlate SignNow, but it is designed to be cost-effective for businesses of all sizes. Pricing plans vary based on features and the number of users, ensuring that you can find a solution that fits your budget. Investing in airSlate SignNow can signNowly enhance your teleconference capabilities when discussing the draft revised plan.
-
Can I integrate airSlate SignNow with other tools for my teleconference?
Absolutely! airSlate SignNow integrates seamlessly with various tools and platforms, enhancing your teleconference experience. Whether you use video conferencing software or project management tools, these integrations allow for a smoother workflow when discussing the draft revised plan. This ensures that all necessary resources are at your fingertips during the call.
-
What are the benefits of using airSlate SignNow during a teleconference?
Using airSlate SignNow during a teleconference provides numerous benefits, including increased efficiency and improved collaboration. Participants can quickly access and sign documents, reducing delays in the decision-making process. This is particularly useful when discussing the draft revised plan, as it allows for immediate feedback and adjustments.
-
How secure is airSlate SignNow for teleconferences?
airSlate SignNow prioritizes security, ensuring that all documents shared during teleconferences are protected. The platform uses advanced encryption and compliance measures to safeguard sensitive information. This level of security is crucial when discussing the draft revised plan, as it builds trust among participants.
Get more for Teleconference, To Discuss In depth The Draft Revised Plan And
- Order interrogatories form
- Intestate succession colorado form
- Colorado llc company 497299759 form
- Quitclaim deed individual to individual colorado form
- Special warranty deed personal representative to individual colorado form
- Colorado beneficiary deed form
- Deed personal representative form
- Transfer death deed form 497299764
Find out other Teleconference, To Discuss In depth The Draft Revised Plan And
- How To Electronic signature Alabama Business Operations Form
- Help Me With Electronic signature Alabama Car Dealer Presentation
- How Can I Electronic signature California Car Dealer PDF
- How Can I Electronic signature California Car Dealer Document
- How Can I Electronic signature Colorado Car Dealer Form
- How To Electronic signature Florida Car Dealer Word
- How Do I Electronic signature Florida Car Dealer Document
- Help Me With Electronic signature Florida Car Dealer Presentation
- Can I Electronic signature Georgia Car Dealer PDF
- How Do I Electronic signature Georgia Car Dealer Document
- Can I Electronic signature Georgia Car Dealer Form
- Can I Electronic signature Idaho Car Dealer Document
- How Can I Electronic signature Illinois Car Dealer Document
- How Can I Electronic signature North Carolina Banking PPT
- Can I Electronic signature Kentucky Car Dealer Document
- Can I Electronic signature Louisiana Car Dealer Form
- How Do I Electronic signature Oklahoma Banking Document
- How To Electronic signature Oklahoma Banking Word
- How Can I Electronic signature Massachusetts Car Dealer PDF
- How Can I Electronic signature Michigan Car Dealer Document