1 Tumor Assessment WorksheetProtocol Subject ID Form


What is the 1 Tumor Assessment WorksheetProtocol Subject ID
The 1 Tumor Assessment WorksheetProtocol Subject ID is a specialized document used primarily in clinical and research settings to collect and assess tumor-related data from subjects. This worksheet is essential for tracking patient information, treatment responses, and tumor characteristics throughout the course of a study. It serves as a standardized tool that ensures consistency in data collection, which is crucial for the validity of clinical trials and research outcomes.
How to use the 1 Tumor Assessment WorksheetProtocol Subject ID
To effectively use the 1 Tumor Assessment WorksheetProtocol Subject ID, follow these steps:
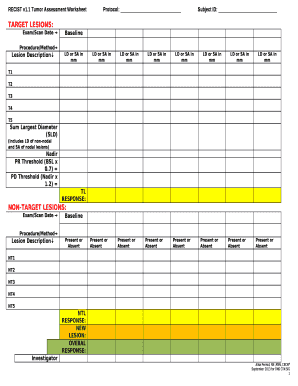
- Begin by filling in the Subject ID, which uniquely identifies each participant.
- Document relevant patient demographic information, including age, gender, and medical history.
- Record tumor characteristics such as type, size, and location, ensuring accuracy for future reference.
- Include treatment details, noting any therapies administered and their corresponding dates.
- Regularly update the worksheet with any changes in the patient's condition or treatment responses.
Steps to complete the 1 Tumor Assessment WorksheetProtocol Subject ID
Completing the 1 Tumor Assessment WorksheetProtocol Subject ID involves several key steps:
- Gather all necessary patient information before starting.
- Fill out the worksheet in a clear and organized manner, ensuring all fields are completed.
- Double-check the accuracy of the data entered, particularly in critical sections like tumor characteristics and treatment history.
- Submit the completed worksheet to the designated research team or clinical coordinator for review.
- Keep a copy of the worksheet for your records and future reference.
Key elements of the 1 Tumor Assessment WorksheetProtocol Subject ID
The 1 Tumor Assessment WorksheetProtocol Subject ID contains several key elements that are crucial for effective data collection:
- Subject Identification: A unique ID for each participant to maintain confidentiality and track individual data.
- Demographics: Basic information about the subject, including age, gender, and ethnicity.
- Tumor Details: Comprehensive information about the tumor, including type, size, grade, and staging.
- Treatment Information: Documentation of all treatments administered, including dates and types of therapies.
- Follow-Up Data: Information on follow-up visits, treatment responses, and any adverse effects observed.
Legal use of the 1 Tumor Assessment WorksheetProtocol Subject ID
The legal use of the 1 Tumor Assessment WorksheetProtocol Subject ID is governed by regulations surrounding patient confidentiality and data protection. It is essential to comply with the Health Insurance Portability and Accountability Act (HIPAA) to ensure that all personal health information is handled appropriately. Researchers and clinical staff must obtain informed consent from participants before collecting data and must ensure that all information is stored securely and used solely for the intended research purposes.
Examples of using the 1 Tumor Assessment WorksheetProtocol Subject ID
Here are a few examples of how the 1 Tumor Assessment WorksheetProtocol Subject ID can be utilized:
- In a clinical trial assessing a new cancer treatment, researchers use the worksheet to track patient responses and side effects over time.
- A hospital oncology department employs the worksheet to standardize data collection for all patients undergoing tumor assessments.
- During a research study on tumor biomarkers, the worksheet helps document the characteristics of tumors from various subjects, facilitating comparative analysis.
Quick guide on how to complete 1 tumor assessment worksheetprotocol subject id
Effortlessly Prepare 1 Tumor Assessment WorksheetProtocol Subject ID on Any Device
Managing documents online has gained traction among businesses and individuals alike. It serves as an ideal eco-friendly alternative to conventional printed and signed paperwork, as you can easily locate the necessary form and securely store it online. airSlate SignNow equips you with all the tools required to create, modify, and electronically sign your documents quickly without delays. Handle 1 Tumor Assessment WorksheetProtocol Subject ID on any device using airSlate SignNow's Android or iOS applications and simplify your document-centric tasks today.
How to Edit and Electronically Sign 1 Tumor Assessment WorksheetProtocol Subject ID with Ease
- Locate 1 Tumor Assessment WorksheetProtocol Subject ID and click on Get Form to begin.
- Utilize the tools we provide to complete your form.
- Emphasize pertinent sections of the documents or redact sensitive information with tools that airSlate SignNow offers specifically for that purpose.
- Create your electronic signature using the Sign feature, which takes mere seconds and holds the same legal validity as a traditional wet ink signature.
- Review the details and click on the Done button to save your changes.
- Select your preferred method to send your form, whether by email, SMS, or invitation link, or download it to your computer.
Say goodbye to lost or misfiled documents, tedious form searches, and mistakes that require printing new copies. airSlate SignNow meets your document management needs with just a few clicks from any device you choose. Modify and electronically sign 1 Tumor Assessment WorksheetProtocol Subject ID and ensure excellent communication throughout the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the 1 tumor assessment worksheetprotocol subject id
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is the 1 Tumor Assessment WorksheetProtocol Subject ID?
The 1 Tumor Assessment WorksheetProtocol Subject ID is a specialized document designed to streamline the assessment process for tumor-related studies. It helps researchers and healthcare professionals efficiently track and manage patient data, ensuring compliance and accuracy in reporting.
-
How does airSlate SignNow support the 1 Tumor Assessment WorksheetProtocol Subject ID?
airSlate SignNow provides a user-friendly platform that allows users to easily create, send, and eSign the 1 Tumor Assessment WorksheetProtocol Subject ID. This ensures that all necessary signatures are obtained quickly, reducing delays in the assessment process.
-
What are the pricing options for using airSlate SignNow with the 1 Tumor Assessment WorksheetProtocol Subject ID?
airSlate SignNow offers flexible pricing plans that cater to various business needs, including options for individual users and teams. By choosing the right plan, you can efficiently manage the 1 Tumor Assessment WorksheetProtocol Subject ID without breaking your budget.
-
What features does airSlate SignNow offer for the 1 Tumor Assessment WorksheetProtocol Subject ID?
Key features of airSlate SignNow include customizable templates, secure eSigning, and real-time tracking of document status. These features enhance the management of the 1 Tumor Assessment WorksheetProtocol Subject ID, making it easier to collaborate and maintain compliance.
-
Can I integrate airSlate SignNow with other tools for the 1 Tumor Assessment WorksheetProtocol Subject ID?
Yes, airSlate SignNow offers seamless integrations with various applications, including CRM systems and project management tools. This allows you to enhance your workflow when handling the 1 Tumor Assessment WorksheetProtocol Subject ID and improve overall efficiency.
-
What are the benefits of using airSlate SignNow for the 1 Tumor Assessment WorksheetProtocol Subject ID?
Using airSlate SignNow for the 1 Tumor Assessment WorksheetProtocol Subject ID provides numerous benefits, including increased efficiency, reduced paperwork, and enhanced security. These advantages help streamline the assessment process and ensure that all data is accurately captured and stored.
-
Is airSlate SignNow secure for handling the 1 Tumor Assessment WorksheetProtocol Subject ID?
Absolutely! airSlate SignNow employs advanced security measures, including encryption and secure cloud storage, to protect sensitive information related to the 1 Tumor Assessment WorksheetProtocol Subject ID. You can trust that your data is safe and compliant with industry standards.
Get more for 1 Tumor Assessment WorksheetProtocol Subject ID
- Kentucky contract for sale and purchase of real estate with no broker for residential home sale agreement form
- Deed of distribution form
- Alienation of affection forms
- Texas disclosure statement required for residential construction contract mechanics liens form
- Limited power of attorney tennessee form
- Nd bill of sale form
- Fulling out quit claim new mexico form
- West virginia fiduciary deed for use by executors trustees trustors administrators and other fiduciaries form
Find out other 1 Tumor Assessment WorksheetProtocol Subject ID
- How To Integrate Sign in Banking
- How To Use Sign in Banking
- Help Me With Use Sign in Banking
- Can I Use Sign in Banking
- How Do I Install Sign in Banking
- How To Add Sign in Banking
- How Do I Add Sign in Banking
- How Can I Add Sign in Banking
- Can I Add Sign in Banking
- Help Me With Set Up Sign in Government
- How To Integrate eSign in Banking
- How To Use eSign in Banking
- How To Install eSign in Banking
- How To Add eSign in Banking
- How To Set Up eSign in Banking
- How To Save eSign in Banking
- How To Implement eSign in Banking
- How To Set Up eSign in Construction
- How To Integrate eSign in Doctors
- How To Use eSign in Doctors