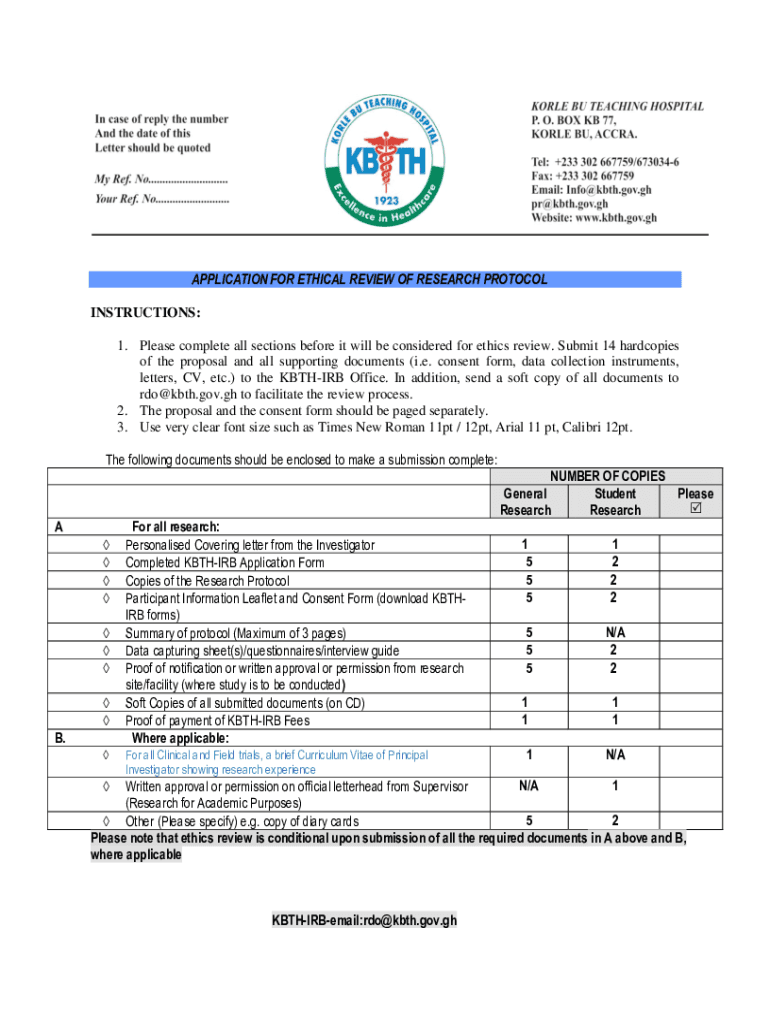
Korle Bu Teaching Hospital Kbth Irb Consent Form


What is the Korle Bu Teaching Hospital Kbth irb Consent Form
The Korle Bu Teaching Hospital Kbth irb Consent Form is a crucial document used in the context of research and clinical studies conducted at the Korle Bu Teaching Hospital in Ghana. This form is designed to ensure that participants are fully informed about the nature of the study, its purpose, potential risks, and benefits before they agree to participate. It serves as a formal agreement between the researchers and the participants, emphasizing the importance of ethical standards in research practices.
How to use the Korle Bu Teaching Hospital Kbth irb Consent Form
Using the Korle Bu Teaching Hospital Kbth irb Consent Form involves several steps to ensure that participants understand their rights and the study's implications. Researchers must provide the form to potential participants, explaining each section clearly. Participants should read the form thoroughly, ask questions if needed, and confirm their understanding before signing. This process helps to foster trust and transparency between researchers and participants, which is essential for ethical research conduct.
Steps to complete the Korle Bu Teaching Hospital Kbth irb Consent Form
Completing the Korle Bu Teaching Hospital Kbth irb Consent Form involves a systematic approach:
- Review the form: Participants should carefully read all sections, including the study's purpose and procedures.
- Ask questions: If any part of the form is unclear, participants are encouraged to seek clarification from the research team.
- Provide information: Participants may need to fill in personal details, such as their name and contact information.
- Sign the form: Once participants understand the study and agree to participate, they should sign and date the form.
This process ensures that participants are fully informed and voluntarily consenting to take part in the research.
Key elements of the Korle Bu Teaching Hospital Kbth irb Consent Form
The Korle Bu Teaching Hospital Kbth irb Consent Form includes several key elements that are vital for informed consent:
- Study Purpose: A clear explanation of why the research is being conducted.
- Procedures: Detailed information on what participants will be asked to do during the study.
- Risks and Benefits: An outline of any potential risks involved and the benefits of participation.
- Confidentiality: Assurance that personal information will be kept confidential and used solely for research purposes.
- Voluntary Participation: A statement emphasizing that participation is voluntary and participants can withdraw at any time without penalty.
Legal use of the Korle Bu Teaching Hospital Kbth irb Consent Form
The Korle Bu Teaching Hospital Kbth irb Consent Form is legally binding and must comply with ethical guidelines and regulations governing research. It is essential for researchers to ensure that the form adheres to the standards set by institutional review boards (IRBs) and relevant legal frameworks. Proper use of this form protects both the rights of participants and the integrity of the research process, ensuring that ethical considerations are met throughout the study.
How to obtain the Korle Bu Teaching Hospital Kbth irb Consent Form
To obtain the Korle Bu Teaching Hospital Kbth irb Consent Form, researchers or participants can follow these steps:
- Contact the research team: Researchers can provide the form directly to potential participants.
- Visit the Korle Bu Teaching Hospital: The hospital may have copies of the form available for distribution.
- Check institutional resources: Some institutions may provide access to the form through their research ethics office or online platforms.
Ensuring access to the form is essential for facilitating informed consent in research studies.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the korle bu teaching hospital kbth irb consent form
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is the Korle Bu Teaching Hospital Kbth irb Consent Form?
The Korle Bu Teaching Hospital Kbth irb Consent Form is a document used to obtain informed consent from participants involved in research studies at the hospital. This form ensures that participants are fully aware of the study's purpose, procedures, risks, and benefits before agreeing to participate.
-
How can airSlate SignNow help with the Korle Bu Teaching Hospital Kbth irb Consent Form?
airSlate SignNow streamlines the process of sending and eSigning the Korle Bu Teaching Hospital Kbth irb Consent Form. With our user-friendly platform, you can easily create, send, and manage consent forms, ensuring compliance and efficiency in your research processes.
-
Is there a cost associated with using airSlate SignNow for the Korle Bu Teaching Hospital Kbth irb Consent Form?
Yes, airSlate SignNow offers various pricing plans to accommodate different needs. Our cost-effective solutions ensure that you can manage the Korle Bu Teaching Hospital Kbth irb Consent Form without breaking your budget, providing excellent value for your investment.
-
What features does airSlate SignNow offer for the Korle Bu Teaching Hospital Kbth irb Consent Form?
airSlate SignNow provides features such as customizable templates, secure eSigning, and real-time tracking for the Korle Bu Teaching Hospital Kbth irb Consent Form. These features enhance the efficiency of document management and ensure that all consent forms are handled securely and professionally.
-
Can I integrate airSlate SignNow with other tools for managing the Korle Bu Teaching Hospital Kbth irb Consent Form?
Absolutely! airSlate SignNow offers integrations with various applications, allowing you to seamlessly manage the Korle Bu Teaching Hospital Kbth irb Consent Form alongside your existing tools. This integration capability enhances your workflow and improves overall productivity.
-
What are the benefits of using airSlate SignNow for the Korle Bu Teaching Hospital Kbth irb Consent Form?
Using airSlate SignNow for the Korle Bu Teaching Hospital Kbth irb Consent Form provides numerous benefits, including increased efficiency, reduced paperwork, and enhanced security. Our platform ensures that your consent forms are processed quickly and securely, allowing you to focus on your research.
-
How secure is the Korle Bu Teaching Hospital Kbth irb Consent Form when using airSlate SignNow?
Security is a top priority at airSlate SignNow. The Korle Bu Teaching Hospital Kbth irb Consent Form is protected with advanced encryption and compliance with industry standards, ensuring that all sensitive information remains confidential and secure throughout the signing process.
Get more for Korle Bu Teaching Hospital Kbth irb Consent Form
- Paternity acknowledgement form 3940 revised 092017
- 2s form
- State of hawaii department of human services hawaiigov form
- Notice of privacy practices mybenefits form
- Provider contact change form hawaii department of health
- Iowa tb tool health form
- Iowa department of human services record check evaluation 2011 form
- Resting respiratory rate dog chart form
Find out other Korle Bu Teaching Hospital Kbth irb Consent Form
- Send Sign PDF Free
- How To Send Sign PDF
- Send Sign Word Online
- Send Sign Word Now
- Send Sign Word Free
- Send Sign Word Android
- Send Sign Word iOS
- Send Sign Word iPad
- How To Send Sign Word
- Can I Send Sign Word
- How Can I Send Sign Word
- Send Sign Document Online
- Send Sign Document Computer
- Send Sign Document Myself
- Send Sign Document Secure
- Send Sign Document iOS
- Send Sign Document iPad
- How To Send Sign Document
- Fax Sign PDF Online
- How To Fax Sign PDF