Register for Schedule H1 Medicines Form


What is the Register For Schedule H1 Medicines
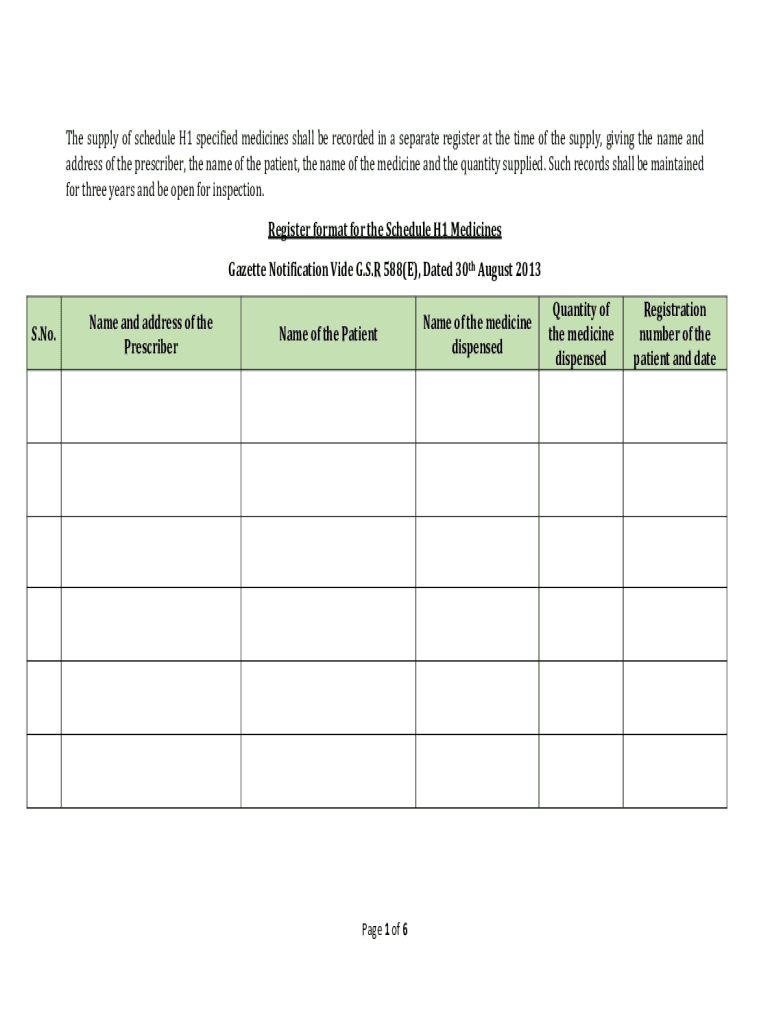
The Register For Schedule H1 Medicines is a formal document required for the regulation and tracking of specific medicinal products classified under Schedule H1. This form is crucial for ensuring compliance with federal and state laws governing the distribution and use of these medicines in the United States. It serves as a means to monitor the handling of substances that may have a significant impact on public health.
How to use the Register For Schedule H1 Medicines
Using the Register For Schedule H1 Medicines involves several steps that ensure proper documentation and compliance. First, individuals or businesses must accurately fill out the form with relevant details regarding the medicines being registered. This includes information about the product, its intended use, and the manufacturer. Once completed, the form must be submitted to the appropriate regulatory body for review and approval.
Steps to complete the Register For Schedule H1 Medicines
Completing the Register For Schedule H1 Medicines requires careful attention to detail. Follow these steps:
- Gather all necessary information about the medicinal product, including its name, composition, and intended use.
- Fill out the form accurately, ensuring all fields are completed to avoid delays.
- Review the form for any errors or omissions before submission.
- Submit the form to the designated regulatory authority, either online or via mail.
Legal use of the Register For Schedule H1 Medicines
The legal use of the Register For Schedule H1 Medicines is governed by various federal and state regulations. It is essential for businesses to adhere to these laws to avoid penalties. The form not only facilitates the legal distribution of Schedule H1 medicines but also protects public health by ensuring that only approved products are available in the market.
Required Documents
To successfully register for Schedule H1 Medicines, certain documents are required. These may include:
- Proof of identity or business registration.
- Detailed product specifications and safety data.
- Any prior approvals or licenses related to the product.
Having these documents ready will streamline the registration process and ensure compliance with regulatory standards.
Filing Deadlines / Important Dates
Staying informed about filing deadlines is crucial for compliance with the Register For Schedule H1 Medicines. Each state may have different deadlines for submission, and it is important to check local regulations. Missing a deadline can result in penalties or delays in obtaining the necessary approvals.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the register for schedule h1 medicines
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is the process to Register For Schedule H1 Medicines?
To Register For Schedule H1 Medicines, you need to complete the online application form on our website. Ensure that you provide all necessary documentation and information required for the registration process. Once submitted, our team will review your application and guide you through the next steps.
-
What are the benefits of Registering For Schedule H1 Medicines?
Registering For Schedule H1 Medicines allows you to access a streamlined process for managing your medicinal products. It ensures compliance with regulatory standards and enhances your business's credibility. Additionally, it opens up opportunities for better market access and customer trust.
-
Are there any fees associated with Registering For Schedule H1 Medicines?
Yes, there are fees associated with Registering For Schedule H1 Medicines, which vary based on the type of registration and the services required. We provide a transparent pricing structure on our website, ensuring you understand all costs involved before proceeding. Contact our support team for detailed pricing information.
-
What features does airSlate SignNow offer for those who Register For Schedule H1 Medicines?
airSlate SignNow offers a range of features designed to simplify the process for those who Register For Schedule H1 Medicines. These include electronic signatures, document templates, and secure storage solutions. Our platform is user-friendly, making it easy to manage your documents efficiently.
-
How does airSlate SignNow ensure compliance when I Register For Schedule H1 Medicines?
When you Register For Schedule H1 Medicines using airSlate SignNow, our platform is designed to comply with all relevant regulations. We implement robust security measures and provide audit trails for all transactions. This ensures that your documentation meets the necessary legal standards.
-
Can I integrate airSlate SignNow with other tools after I Register For Schedule H1 Medicines?
Absolutely! After you Register For Schedule H1 Medicines, you can easily integrate airSlate SignNow with various tools and applications. Our platform supports integrations with popular software like CRM systems, project management tools, and more, enhancing your workflow efficiency.
-
What support is available if I have questions about Registering For Schedule H1 Medicines?
We offer comprehensive support for anyone looking to Register For Schedule H1 Medicines. Our customer service team is available via chat, email, or phone to assist you with any questions or concerns. Additionally, we provide a detailed FAQ section and resources on our website.
Get more for Register For Schedule H1 Medicines
Find out other Register For Schedule H1 Medicines
- eSign Colorado Banking Rental Application Online
- Can I eSign Colorado Banking Medical History
- eSign Connecticut Banking Quitclaim Deed Free
- eSign Connecticut Banking Business Associate Agreement Secure
- Sign Georgia Courts Moving Checklist Simple
- Sign Georgia Courts IOU Mobile
- How Can I Sign Georgia Courts Lease Termination Letter
- eSign Hawaii Banking Agreement Simple
- eSign Hawaii Banking Rental Application Computer
- eSign Hawaii Banking Agreement Easy
- eSign Hawaii Banking LLC Operating Agreement Fast
- eSign Hawaii Banking Permission Slip Online
- eSign Minnesota Banking LLC Operating Agreement Online
- How Do I eSign Mississippi Banking Living Will
- eSign New Jersey Banking Claim Mobile
- eSign New York Banking Promissory Note Template Now
- eSign Ohio Banking LLC Operating Agreement Now
- Sign Maryland Courts Quitclaim Deed Free
- How To Sign Massachusetts Courts Quitclaim Deed
- Can I Sign Massachusetts Courts Quitclaim Deed