Kidney Clinical Research & Epidemiology NIDDK NIH 2015-2026


What is the Kidney Clinical Research & Epidemiology NIDDK NIH
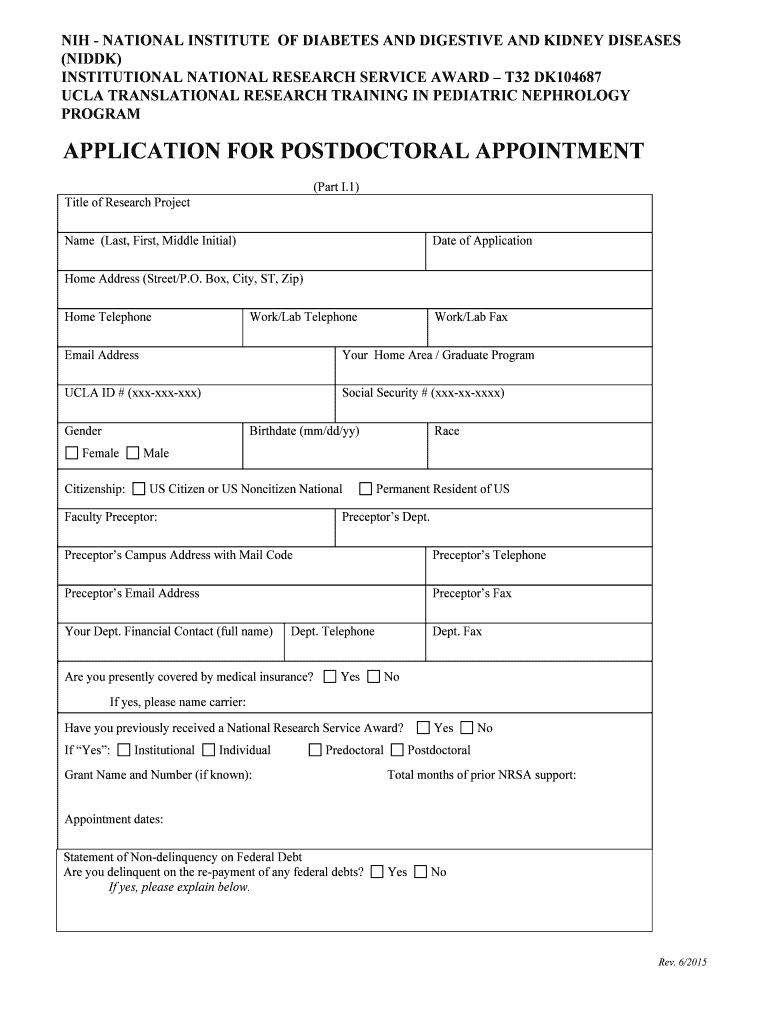
The Kidney Clinical Research & Epidemiology program, under the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) at the National Institutes of Health (NIH), focuses on advancing the understanding of kidney diseases. This program conducts extensive research to identify risk factors, improve treatment options, and enhance patient outcomes. By analyzing data from diverse populations, the initiative aims to address disparities in kidney health and promote effective interventions.
How to use the Kidney Clinical Research & Epidemiology NIDDK NIH
Using the Kidney Clinical Research & Epidemiology NIDDK NIH form involves several steps to ensure accurate data collection and compliance with research protocols. Participants typically need to provide personal health information, medical history, and consent for data usage. It is essential to follow the instructions provided with the form carefully, ensuring that all sections are completed thoroughly to facilitate the research process.
Steps to complete the Kidney Clinical Research & Epidemiology NIDDK NIH
Completing the Kidney Clinical Research & Epidemiology NIDDK NIH form requires attention to detail. Here are the key steps:
- Read the instructions carefully to understand the requirements.
- Gather necessary personal and medical information before starting.
- Fill out the form accurately, ensuring all fields are completed.
- Review the form for any errors or omissions.
- Submit the form electronically or as directed in the instructions.
Legal use of the Kidney Clinical Research & Epidemiology NIDDK NIH
To ensure the legal use of the Kidney Clinical Research & Epidemiology NIDDK NIH form, compliance with federal regulations regarding health information is crucial. This includes adherence to the Health Insurance Portability and Accountability Act (HIPAA), which protects patient privacy. Additionally, obtaining informed consent from participants is necessary to validate the use of their data in research.
Key elements of the Kidney Clinical Research & Epidemiology NIDDK NIH
The Kidney Clinical Research & Epidemiology NIDDK NIH form includes several key elements that are vital for research integrity. These elements typically encompass:
- Personal identification information.
- Medical history and current health status.
- Consent for data collection and usage.
- Demographic information to ensure diverse representation.
Examples of using the Kidney Clinical Research & Epidemiology NIDDK NIH
Examples of utilizing the Kidney Clinical Research & Epidemiology NIDDK NIH form can be seen in various research studies aimed at understanding kidney disease prevalence. For instance, researchers may analyze data collected to identify trends in kidney health among different age groups or ethnicities. This information can lead to targeted health interventions and policies that improve kidney health outcomes across populations.
Quick guide on how to complete kidney clinical research ampamp epidemiology niddk nih
Effortlessly Prepare Kidney Clinical Research & Epidemiology NIDDK NIH on Any Device
Online document management has become popular among organizations and individuals. It offers an excellent eco-friendly alternative to traditional printed and signed documents, enabling you to access the appropriate form and securely store it online. airSlate SignNow provides you with all the tools necessary to create, edit, and eSign your documents swiftly without delays. Manage Kidney Clinical Research & Epidemiology NIDDK NIH on any platform with airSlate SignNow's Android or iOS applications and simplify any document-related task today.
The Easiest Way to Edit and eSign Kidney Clinical Research & Epidemiology NIDDK NIH with Ease
- Find Kidney Clinical Research & Epidemiology NIDDK NIH and click on Get Form to begin.
- Use the tools we offer to complete your form.
- Highlight important sections of the documents or redact sensitive information with tools specially designed by airSlate SignNow for this purpose.
- Create your eSignature using the Sign feature, which takes only seconds and carries the same legal validity as a traditional handwritten signature.
- Review all the information and click on the Done button to save your modifications.
- Select your preferred method for providing your form, whether by email, SMS, invite link, or downloading it to your computer.
Eliminate worries about lost or misplaced files, tedious form searches, or errors that require printing new copies of documents. airSlate SignNow addresses all your document management needs in just a few clicks from any device you choose. Edit and eSign Kidney Clinical Research & Epidemiology NIDDK NIH while ensuring excellent communication throughout your form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the kidney clinical research ampamp epidemiology niddk nih
The best way to create an eSignature for a PDF file in the online mode
The best way to create an eSignature for a PDF file in Chrome
The best way to create an electronic signature for putting it on PDFs in Gmail
How to generate an electronic signature straight from your smartphone
The way to generate an eSignature for a PDF file on iOS devices
How to generate an electronic signature for a PDF document on Android
People also ask
-
What is the purpose of Kidney Clinical Research & Epidemiology NIDDK NIH?
Kidney Clinical Research & Epidemiology NIDDK NIH aims to advance the understanding of kidney diseases and improve patient outcomes through rigorous scientific research. By focusing on clinical studies and epidemiological data, it contributes signNowly to public health initiatives and promotes kidney health awareness.
-
How does airSlate SignNow support research documentation in Kidney Clinical Research & Epidemiology NIDDK NIH?
airSlate SignNow provides a user-friendly platform to easily create, send, and eSign documents essential for Kidney Clinical Research & Epidemiology NIDDK NIH. This enhances collaboration among researchers and ensures compliance and security of sensitive research documentation.
-
What are the pricing options for using airSlate SignNow in clinical research?
AirSlate SignNow offers flexible pricing plans suitable for organizations involved in Kidney Clinical Research & Epidemiology NIDDK NIH. Whether you’re a small research team or a large institute, there’s a cost-effective solution tailored to fit your document management needs and budget.
-
Can airSlate SignNow integrate with other research management tools?
Yes, airSlate SignNow provides seamless integrations with various research management tools and platforms commonly used in Kidney Clinical Research & Epidemiology NIDDK NIH. This enables researchers to streamline their workflows and enhance data sharing across existing systems.
-
What benefits does airSlate SignNow offer for clinical trials?
AirSlate SignNow enhances the efficiency of clinical trials in Kidney Clinical Research & Epidemiology NIDDK NIH by reducing paperwork and expediting the eSigning process. This ensures that trial documentation is processed quickly, supporting faster research cycles and improving data accuracy.
-
Is airSlate SignNow secure for handling sensitive research data?
Absolutely! airSlate SignNow employs robust security measures to protect sensitive data relevant to Kidney Clinical Research & Epidemiology NIDDK NIH. With features like encryption and compliance with legal standards, you can be assured that your research documents are safeguarded.
-
How can airSlate SignNow help with participant consent forms?
AirSlate SignNow simplifies the collection and management of participant consent forms in studies related to Kidney Clinical Research & Epidemiology NIDDK NIH. The platform allows for efficient electronic signatures, ensuring that consent is obtained and documented effectively.
Get more for Kidney Clinical Research & Epidemiology NIDDK NIH
- P 321 order starting formal probate and appointing pr yes will 8
- Tf 700 form
- Alaska tr 218 form
- Dr 130 request to modify decree of dissolution order 10 15 domestic relations form
- Pg 800 petition for protection from financial abuse 10 15 combo pg 827 law enforcement sheet 7 12 fill in form
- Tf410 form pdf
- Cn 350 alaska court records state of alaska form
- Affidavit proof jail facility form
Find out other Kidney Clinical Research & Epidemiology NIDDK NIH
- Can I eSignature Oregon Orthodontists LLC Operating Agreement
- How To eSignature Rhode Island Orthodontists LLC Operating Agreement
- Can I eSignature West Virginia Lawers Cease And Desist Letter
- eSignature Alabama Plumbing Confidentiality Agreement Later
- How Can I eSignature Wyoming Lawers Quitclaim Deed
- eSignature California Plumbing Profit And Loss Statement Easy
- How To eSignature California Plumbing Business Letter Template
- eSignature Kansas Plumbing Lease Agreement Template Myself
- eSignature Louisiana Plumbing Rental Application Secure
- eSignature Maine Plumbing Business Plan Template Simple
- Can I eSignature Massachusetts Plumbing Business Plan Template
- eSignature Mississippi Plumbing Emergency Contact Form Later
- eSignature Plumbing Form Nebraska Free
- How Do I eSignature Alaska Real Estate Last Will And Testament
- Can I eSignature Alaska Real Estate Rental Lease Agreement
- eSignature New Jersey Plumbing Business Plan Template Fast
- Can I eSignature California Real Estate Contract
- eSignature Oklahoma Plumbing Rental Application Secure
- How Can I eSignature Connecticut Real Estate Quitclaim Deed
- eSignature Pennsylvania Plumbing Business Plan Template Safe