Global Adverse Event and Special Situation Reporting Roche Pro Form


Key elements of the Global Adverse Event And Special Situation Reporting
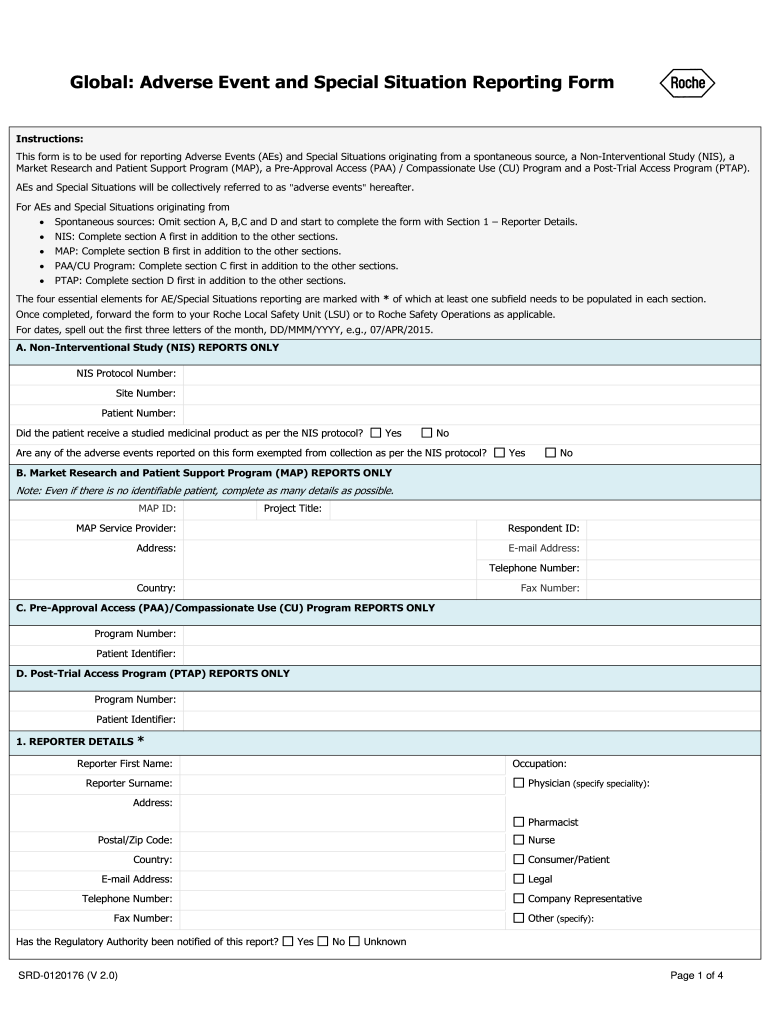
The Global Adverse Event and Special Situation Reporting form is designed to capture critical information regarding adverse events and special situations. Key elements include:
- Patient Information: Details such as the patient's name, age, and medical history are essential for context.
- Event Description: A clear and concise account of the adverse event, including symptoms, severity, and duration.
- Reporting Source: Identification of the individual or organization reporting the event, along with their contact information.
- Outcome: Information on the resolution of the event, including any medical interventions and patient recovery status.
- Date and Time: Accurate timestamps for when the event occurred and when it was reported are crucial for tracking trends.
Steps to complete the Global Adverse Event And Special Situation Reporting
Completing the Global Adverse Event and Special Situation Reporting form involves a systematic approach to ensure accuracy and compliance. The following steps can guide you through the process:
- Gather Information: Collect all relevant patient and event details before starting the form.
- Fill Out the Form: Carefully input the information into the designated fields, ensuring clarity and precision.
- Review for Accuracy: Double-check all entries for correctness, including spelling and numerical values.
- Submit the Form: Follow the specified submission method, whether online or by mail, ensuring it reaches the appropriate authority.
- Keep a Copy: Retain a copy of the completed form for your records, which may be necessary for future reference.
Legal use of the Global Adverse Event And Special Situation Reporting
The legal use of the Global Adverse Event and Special Situation Reporting form is governed by regulations that ensure the protection of patient information and the integrity of the reporting process. Compliance with laws such as HIPAA and other relevant privacy regulations is essential. The form must be filled out accurately and submitted in a timely manner to avoid legal repercussions. Additionally, organizations should maintain secure records of all submissions to demonstrate compliance during audits.
Examples of using the Global Adverse Event And Special Situation Reporting
Understanding how to effectively utilize the Global Adverse Event and Special Situation Reporting form can enhance reporting practices. Here are a few examples:
- Clinical Trials: Researchers may report adverse events encountered during drug trials to ensure participant safety and regulatory compliance.
- Post-Marketing Surveillance: Pharmaceutical companies are required to report any adverse events related to their products after they have been approved for public use.
- Healthcare Facilities: Hospitals and clinics must report any significant adverse events to regulatory bodies to maintain transparency and improve patient care.
How to use the Global Adverse Event And Special Situation Reporting
Using the Global Adverse Event and Special Situation Reporting form effectively involves understanding its purpose and the required information. Start by familiarizing yourself with the form layout and the specific sections. Each section is designed to capture vital information, so take your time to provide detailed responses. Utilizing digital tools can streamline the process, allowing for easier data entry and submission. Ensure that all stakeholders involved in the reporting process are trained on the form's use to enhance compliance and accuracy.
State-specific rules for the Global Adverse Event And Special Situation Reporting
State-specific rules for the Global Adverse Event and Special Situation Reporting may vary, reflecting local regulations and reporting requirements. It is important to be aware of these variations to ensure compliance. For instance, some states may have additional reporting criteria or deadlines that must be adhered to. Always consult state health department guidelines or legal resources to stay informed about any specific obligations that apply to your location.
Quick guide on how to complete global adverse event and special situation reporting roche pro
Effortlessly Prepare Global Adverse Event And Special Situation Reporting Roche Pro on Any Device
Digital document management has gained traction among businesses and individuals alike. It offers an excellent environmentally friendly substitute for traditional printed and signed paperwork, allowing you to locate the necessary template and securely keep it online. airSlate SignNow equips you with all the resources needed to create, edit, and electronically sign your documents swiftly without delays. Handle Global Adverse Event And Special Situation Reporting Roche Pro on any device using the airSlate SignNow apps for Android or iOS and simplify your document-related tasks today.
The Easiest Way to Edit and Electronically Sign Global Adverse Event And Special Situation Reporting Roche Pro Effortlessly
- Obtain Global Adverse Event And Special Situation Reporting Roche Pro and click Get Form to begin.
- Utilize the tools provided to fill out your form.
- Emphasize relevant sections of the documents or obscure sensitive information with the specific tools that airSlate SignNow offers.
- Create your electronic signature using the Sign feature, which takes mere seconds and carries the same legal standing as a conventional wet ink signature.
- Review the details and click on the Done button to save your changes.
- Choose your preferred method of sending the form, whether by email, SMS, or invitation link, or download it to your computer.
Eliminate the hassle of lost or misplaced documents, tedious form searching, or errors that necessitate printing new copies. airSlate SignNow fulfills all your document management needs within a few clicks from any device you prefer. Update and electronically sign Global Adverse Event And Special Situation Reporting Roche Pro and ensure effective communication at every stage of the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the global adverse event and special situation reporting roche pro
How to create an electronic signature for a PDF file online
How to create an electronic signature for a PDF file in Google Chrome
How to create an electronic signature for signing PDFs in Gmail
The way to create an eSignature right from your mobile device
How to create an eSignature for a PDF file on iOS
The way to create an eSignature for a PDF on Android devices
People also ask
-
What is a situational report sample?
A situational report sample is a template that outlines specific information about an event or occurrence. It typically includes data about the current situation, key stakeholders, and actionable insights. Using a situational report sample helps organizations standardize their reporting process and improve communication.
-
How can airSlate SignNow assist in creating situational report samples?
airSlate SignNow provides a seamless platform for creating and sharing situational report samples electronically. Our intuitive interface allows you to customize your reports and easily add electronic signatures for approvals. This speeds up the documentation process and ensures all stakeholders are aligned.
-
Are there any costs associated with using situational report samples on airSlate SignNow?
airSlate SignNow offers competitive pricing plans that accommodate various business needs, including access to situational report samples. Customers can choose from several subscription options, ensuring they find a plan that fits their budget while benefiting from all the features we offer.
-
What features does airSlate SignNow offer for managing situational report samples?
Key features of airSlate SignNow include customizable templates for situational reports, secure eSigning capabilities, and real-time collaboration tools. You can share your situational report samples with team members easily and track their completion status. These features enhance productivity and streamline your document workflow.
-
What are the benefits of using situational report samples in airSlate SignNow?
Using situational report samples in airSlate SignNow offers several benefits, including increased efficiency, reduced paperwork, and improved accuracy in reporting. It allows teams to create consistent documentation that is easily accessible and modifiable. This ensures that everyone is on the same page, which is critical during critical situations.
-
Can I integrate airSlate SignNow with other tools to manage situational report samples?
Yes, airSlate SignNow supports integration with various popular business tools, allowing you to manage your situational report samples seamlessly. You can connect it with project management software, email services, and more. This ensures that your workflow remains uninterrupted while maintaining access to vital document management features.
-
Is airSlate SignNow secure for handling sensitive situational report samples?
Absolutely! airSlate SignNow prioritizes security with advanced encryption and compliance with industry standards. Your situational report samples are securely stored and protected, ensuring that sensitive information remains confidential. Trust us to safeguard your important documents while you focus on making informed decisions.
Get more for Global Adverse Event And Special Situation Reporting Roche Pro
- Framing contract for contractor missouri form
- Security contract for contractor missouri form
- Insulation contract for contractor missouri form
- Paving contract for contractor missouri form
- Site work contract for contractor missouri form
- Siding contract for contractor missouri form
- Refrigeration contract for contractor missouri form
- Missouri drainage form
Find out other Global Adverse Event And Special Situation Reporting Roche Pro
- How To Integrate Sign in Banking
- How To Use Sign in Banking
- Help Me With Use Sign in Banking
- Can I Use Sign in Banking
- How Do I Install Sign in Banking
- How To Add Sign in Banking
- How Do I Add Sign in Banking
- How Can I Add Sign in Banking
- Can I Add Sign in Banking
- Help Me With Set Up Sign in Government
- How To Integrate eSign in Banking
- How To Use eSign in Banking
- How To Install eSign in Banking
- How To Add eSign in Banking
- How To Set Up eSign in Banking
- How To Save eSign in Banking
- How To Implement eSign in Banking
- How To Set Up eSign in Construction
- How To Integrate eSign in Doctors
- How To Use eSign in Doctors