DAIT Pharmacy Guidelines Investigational Product Accountability Record Form DAIT Pharmacy Guidelines Investigational Product Acc


Understanding the DAIT Pharmacy Guidelines Investigational Product Accountability Record Form
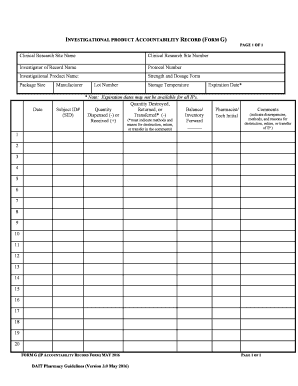
The DAIT Pharmacy Guidelines Investigational Product Accountability Record Form is a crucial document used in the management and tracking of investigational products in clinical trials. This form ensures that all aspects of product accountability are documented, including receipt, storage, dispensing, and return of investigational products. It is essential for maintaining compliance with regulatory standards and for facilitating audits by oversight bodies.
Steps to Complete the DAIT Pharmacy Guidelines Investigational Product Accountability Record Form
Completing the DAIT Pharmacy Guidelines Investigational Product Accountability Record Form involves several key steps:
- Gather Required Information: Collect all necessary details about the investigational product, including its name, batch number, and expiration date.
- Document Receipt: Record the date of receipt and the quantity of the investigational product received.
- Track Usage: Maintain accurate records of how much product is dispensed to participants and any returns.
- Ensure Compliance: Verify that all entries are made in accordance with regulatory guidelines and institutional policies.
- Review and Sign: Have the form reviewed and signed by authorized personnel to validate the accuracy of the information provided.
Legal Use of the DAIT Pharmacy Guidelines Investigational Product Accountability Record Form
The legal use of the DAIT Pharmacy Guidelines Investigational Product Accountability Record Form hinges on compliance with federal regulations. This form must be filled out accurately to ensure that the investigational product is handled according to the guidelines set forth by the FDA and other regulatory agencies. Proper documentation serves as a legal record that can be referenced during audits or in case of disputes regarding product management.
Key Elements of the DAIT Pharmacy Guidelines Investigational Product Accountability Record Form
The key elements of the DAIT Pharmacy Guidelines Investigational Product Accountability Record Form include:
- Product Identification: Clear identification of the investigational product, including its name and unique identifiers.
- Accountability Records: Detailed logs of product storage conditions, dispensing records, and participant information.
- Compliance Statements: Sections that affirm adherence to regulatory requirements and institutional policies.
- Signatures: Required signatures from authorized personnel to confirm the accuracy of the information.
How to Obtain the DAIT Pharmacy Guidelines Investigational Product Accountability Record Form
The DAIT Pharmacy Guidelines Investigational Product Accountability Record Form can typically be obtained through institutional research offices or pharmacy departments involved in clinical trials. Additionally, regulatory bodies may provide templates or guidelines for the form. It is important to ensure that the version used is the most current to comply with updated regulations.
Examples of Using the DAIT Pharmacy Guidelines Investigational Product Accountability Record Form
Examples of using the DAIT Pharmacy Guidelines Investigational Product Accountability Record Form include:
- Clinical Trials: Documenting the accountability of investigational products during Phase I, II, or III clinical trials.
- Research Studies: Tracking the use of investigational products in various research settings to ensure compliance with study protocols.
- Regulatory Audits: Providing a comprehensive record during audits by regulatory agencies to demonstrate proper handling and accountability.
Quick guide on how to complete dait pharmacy guidelines investigational product accountability record form dait pharmacy guidelines investigational product
Access DAIT Pharmacy Guidelines Investigational Product Accountability Record Form DAIT Pharmacy Guidelines Investigational Product Acc effortlessly on any device
Digital document management has become increasingly popular among businesses and individuals. It offers an ideal eco-friendly substitute for traditional printed and signed documents, as you can easily locate the right form and securely keep it online. airSlate SignNow provides you with all the resources necessary to create, modify, and electronically sign your documents quickly without holdups. Manage DAIT Pharmacy Guidelines Investigational Product Accountability Record Form DAIT Pharmacy Guidelines Investigational Product Acc on any platform using airSlate SignNow's Android or iOS applications and streamline any document-related process today.
The simplest way to modify and eSign DAIT Pharmacy Guidelines Investigational Product Accountability Record Form DAIT Pharmacy Guidelines Investigational Product Acc seamlessly
- Find DAIT Pharmacy Guidelines Investigational Product Accountability Record Form DAIT Pharmacy Guidelines Investigational Product Acc and click on Get Form to begin.
- Utilize the tools we offer to fill out your form.
- Emphasize pertinent sections of your documents or obscure sensitive information with tools that airSlate SignNow provides specifically for that purpose.
- Create your electronic signature using the Sign feature, which takes mere seconds and carries the same legal validity as a physical ink signature.
- Review all the details and click on the Done button to finalize your changes.
- Choose how you want to send your form, via email, text (SMS), invitation link, or download it to your computer.
Eliminate concerns about lost or misplaced files, tedious form searches, or errors that necessitate printing new document copies. airSlate SignNow addresses all your needs in document management with just a few clicks on your preferred device. Modify and eSign DAIT Pharmacy Guidelines Investigational Product Accountability Record Form DAIT Pharmacy Guidelines Investigational Product Acc to ensure effective communication at every stage of the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
People also ask
-
What are the dait pharmacy guidelines for document management?
The dait pharmacy guidelines focus on ensuring the proper management and security of pharmaceutical documents. By using airSlate SignNow, businesses can adhere to these guidelines through secure eSigning and document storage. Our platform simplifies compliance, making it easier for pharmacies to manage their workflow.
-
How does airSlate SignNow help with the dait pharmacy guidelines?
airSlate SignNow provides features that align with the dait pharmacy guidelines, including secure eSignature and document tracking. This helps pharmacies maintain compliance with industry standards while streamlining their document handling processes. With our solution, you can ensure that all documentation is properly signed and stored.
-
What pricing options are available for airSlate SignNow to meet the dait pharmacy guidelines?
airSlate SignNow offers a variety of pricing plans to accommodate different business sizes, all designed to support compliance with dait pharmacy guidelines. Our competitive pricing ensures that you can access essential eSigning features at an affordable cost. Contact us for a customized plan that suits your pharmacy's needs.
-
Can airSlate SignNow integrate with existing systems to support dait pharmacy guidelines?
Yes, airSlate SignNow offers seamless integrations with various pharmacy management systems to help fulfill the dait pharmacy guidelines effectively. Our platform can connect with your existing software to streamline document workflows and enhance compliance. This integration simplifies the process of managing pharmacy documentation.
-
What are the key features of airSlate SignNow that support the dait pharmacy guidelines?
Key features of airSlate SignNow that align with the dait pharmacy guidelines include secure eSigning, document templates, and real-time tracking. These tools provide pharmacies with a robust solution for managing their documentation securely and efficiently. By utilizing these features, you can enhance your compliance while saving time and resources.
-
How can airSlate SignNow improve workflow for pharmacies adhering to dait pharmacy guidelines?
airSlate SignNow improves workflow for pharmacies by providing an intuitive platform for document creation and management aligned with dait pharmacy guidelines. Our easy-to-use interface reduces administrative burdens and increases efficiency in document handling. This allows pharmacists to focus more on patient care rather than paperwork.
-
Is training available for pharmacies implementing the dait pharmacy guidelines with airSlate SignNow?
Yes, airSlate SignNow offers comprehensive training and support for pharmacies to facilitate the implementation of dait pharmacy guidelines. Our dedicated support team is available to assist with onboarding, ensuring your staff is well-versed in using our platform. Documentation and resources are also provided to make the transition smooth.
Get more for DAIT Pharmacy Guidelines Investigational Product Accountability Record Form DAIT Pharmacy Guidelines Investigational Product Acc
- Patient prescriber agreement form
- Baptism certificate african methodist episcopal church baptism certificate african methodist episcopal church form
- Systematic observation form
- Canine health record two colors kennel form
- Request for title commitment form
- Cancer type id form
- Sol no dues form
- Business tax missouri department of revenue mo gov form
Find out other DAIT Pharmacy Guidelines Investigational Product Accountability Record Form DAIT Pharmacy Guidelines Investigational Product Acc
- How Can I eSignature Wisconsin Orthodontists Word
- How Do I eSignature Arizona Real Estate PDF
- How To eSignature Arkansas Real Estate Document
- How Do I eSignature Oregon Plumbing PPT
- How Do I eSignature Connecticut Real Estate Presentation
- Can I eSignature Arizona Sports PPT
- How Can I eSignature Wisconsin Plumbing Document
- Can I eSignature Massachusetts Real Estate PDF
- How Can I eSignature New Jersey Police Document
- How Can I eSignature New Jersey Real Estate Word
- Can I eSignature Tennessee Police Form
- How Can I eSignature Vermont Police Presentation
- How Do I eSignature Pennsylvania Real Estate Document
- How Do I eSignature Texas Real Estate Document
- How Can I eSignature Colorado Courts PDF
- Can I eSignature Louisiana Courts Document
- How To Electronic signature Arkansas Banking Document
- How Do I Electronic signature California Banking Form
- How Do I eSignature Michigan Courts Document
- Can I eSignature Missouri Courts Document