Nda Submission Checklist Form


What is the NDA submission checklist?
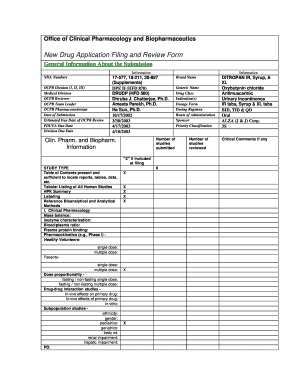
The NDA submission checklist is a comprehensive guide designed to assist businesses in preparing and submitting a New Drug Application (NDA) to the FDA. This checklist ensures that all necessary components are included, facilitating a smoother review process. It typically includes requirements such as clinical study data, manufacturing information, labeling, and safety assessments. By following this checklist, applicants can enhance the likelihood of a successful submission, ensuring compliance with FDA regulations.
Key elements of the NDA submission checklist
Understanding the key elements of the NDA submission checklist is crucial for a successful application. Important components include:
- Clinical data: Comprehensive results from clinical trials demonstrating the drug's safety and efficacy.
- Manufacturing details: Information on the drug's production process, including quality control measures.
- Labeling: Proposed labeling that meets FDA requirements, including dosage, administration, and safety information.
- Preclinical data: Results from laboratory and animal studies that support the drug's safety profile.
- Risk evaluation: Analysis of potential risks associated with the drug and proposed mitigation strategies.
Steps to complete the NDA submission checklist
Completing the NDA submission checklist involves several critical steps to ensure thorough preparation:
- Gather all necessary clinical and preclinical data.
- Compile manufacturing information, including processes and quality control measures.
- Draft the proposed labeling in compliance with FDA guidelines.
- Conduct a thorough review to ensure all components meet FDA standards.
- Submit the completed checklist along with the NDA application to the FDA.
How to use the NDA submission checklist
Utilizing the NDA submission checklist effectively requires a systematic approach. Start by reviewing each item on the checklist to identify what is needed for your specific application. As you gather documents and data, check them off to ensure nothing is overlooked. Regularly consult the checklist throughout the preparation process to stay organized and on track. This proactive approach can significantly reduce the risk of delays in the FDA review process.
Legal use of the NDA submission checklist
The NDA submission checklist serves a legal purpose by ensuring that all required information is provided to the FDA for regulatory review. Adhering to the checklist helps applicants comply with federal regulations, reducing the likelihood of non-compliance penalties. It is essential to understand that incomplete submissions can lead to delays or rejections, making the checklist a vital tool for legal compliance in the drug approval process.
Required documents for the NDA submission checklist
Several documents are essential for a complete NDA submission checklist. These typically include:
- Clinical trial reports and summaries.
- Manufacturing process documentation and quality assurance records.
- Proposed labeling and packaging information.
- Preclinical study reports.
- Risk management plans.
Quick guide on how to complete nda submission
Complete nda submission effortlessly on any device
Digital document management has become favored by businesses and individuals alike. It offers an ideal eco-conscious alternative to traditional printed and signed documents, allowing you to access the correct format and securely store it online. airSlate SignNow provides you with all the tools necessary to create, edit, and eSign your documents swiftly without any hold-ups. Handle nda checklist on any device using airSlate SignNow's Android or iOS applications and enhance any document-oriented workflow today.
The simplest method to alter and eSign nda review checklist with ease
- Find nda checklist fda and click on Get Form to begin.
- Utilize the tools we offer to complete your document.
- Emphasize relevant sections of the documents or redact sensitive details using tools that airSlate SignNow provides specifically for this purpose.
- Create your eSignature with the Sign feature, which takes mere seconds and carries the same legal validity as a conventional wet ink signature.
- Review all the details and click on the Done button to save your modifications.
- Choose how you want to deliver your form—via email, SMS, or invitation link—or download it to your computer.
Eliminate concerns about lost or misplaced documents, tedious form searches, or mistakes that necessitate printing new document copies. airSlate SignNow meets your document management requirements in just a few clicks from any device you prefer. Modify and eSign electronic nda submission and ensure superior communication at every phase of the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Related searches to nda checklist
Create this form in 5 minutes!
How to create an eSignature for the nda review checklist
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask electronic nda submission
-
What is an NDA checklist?
An NDA checklist is a comprehensive guide that outlines the essential elements needed to create a non-disclosure agreement. It ensures that all critical components are included to protect confidential information effectively. Using an NDA checklist helps streamline the drafting process and minimizes potential legal issues.
-
How can airSlate SignNow assist with my NDA checklist?
airSlate SignNow provides tools that simplify the creation and management of NDAs. Our platform allows you to upload your NDA checklist and customize it according to your business needs. You can easily send documents for eSigning, ensuring all parties complete the checklist requirements effortlessly.
-
Is there a cost associated with using airSlate SignNow for my NDA checklist?
Yes, airSlate SignNow offers various pricing plans to suit your needs. Depending on the features you choose, our plans are cost-effective while providing robust functionalities to manage your NDA checklist and other documents. You can explore our pricing page for detailed information.
-
What features does airSlate SignNow offer for managing NDA checklists?
airSlate SignNow includes features like customizable templates, secure eSigning, and collaborative tools, all of which enhance how you manage your NDA checklist. Additionally, our platform supports real-time tracking and reminders, ensuring that all necessary steps are completed promptly.
-
Can I integrate airSlate SignNow with other tools for my NDA checklist?
Absolutely! airSlate SignNow offers integrations with various platforms, enabling you to streamline your workflow while managing your NDA checklist. Whether you're using CRM systems, project management tools, or others, our integrations simplify documentation processes across different platforms.
-
What are the benefits of using an NDA checklist in airSlate SignNow?
Using an NDA checklist in airSlate SignNow helps ensure that you don't miss any critical steps in the signing process. It enhances clarity, builds trust between parties, and provides a legal framework that protects sensitive information. This systematic approach not only saves time but also reduces the risk of disputes.
-
How secure is the information in my NDA checklist with airSlate SignNow?
Security is a top priority for airSlate SignNow. All documents, including your NDA checklist, are encrypted and stored securely on our platform. We comply with industry standards to protect your sensitive information and ensure that only authorized individuals have access to it.
Get more for nda submission
- An adventure into cells and their parts form
- Driveway construction self assessable notification moreton bay bb form
- Budget and decision form 901 25
- Gambinos pizza application for employment form
- Pc 505 wisconsin form
- Boston public schools physical form
- Bofi atm debit card dispute affidavit bank of internet usa form
- Vacation bible school invitation template form
Find out other nda submission checklist
- Electronic signature Alabama Disclosure Notice Simple
- Electronic signature Massachusetts Disclosure Notice Free
- Electronic signature Delaware Drug Testing Consent Agreement Easy
- Electronic signature North Dakota Disclosure Notice Simple
- Electronic signature California Car Lease Agreement Template Free
- How Can I Electronic signature Florida Car Lease Agreement Template
- Electronic signature Kentucky Car Lease Agreement Template Myself
- Electronic signature Texas Car Lease Agreement Template Easy
- Electronic signature New Mexico Articles of Incorporation Template Free
- Electronic signature New Mexico Articles of Incorporation Template Easy
- Electronic signature Oregon Articles of Incorporation Template Simple
- eSignature Montana Direct Deposit Enrollment Form Easy
- How To Electronic signature Nevada Acknowledgement Letter
- Electronic signature New Jersey Acknowledgement Letter Free
- Can I eSignature Oregon Direct Deposit Enrollment Form
- Electronic signature Colorado Attorney Approval Later
- How To Electronic signature Alabama Unlimited Power of Attorney
- Electronic signature Arizona Unlimited Power of Attorney Easy
- Can I Electronic signature California Retainer Agreement Template
- How Can I Electronic signature Missouri Unlimited Power of Attorney