HIV Point of Care Test INSTI Quality Control Log Form


Understanding the HIV Point of Care Test INSTI Quality Control Log
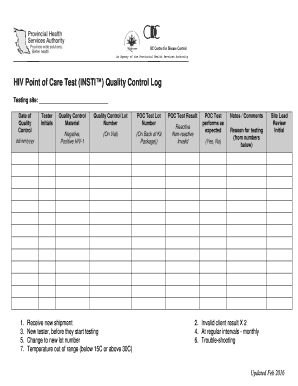
The HIV Point of Care Test INSTI Quality Control Log is a crucial document used to ensure the accuracy and reliability of HIV testing results. This log serves as a record of quality control measures taken during the testing process, which is essential for maintaining compliance with regulatory standards. It typically includes information such as the date of testing, the control results, and any corrective actions taken if results fall outside acceptable ranges. Maintaining this log helps healthcare providers demonstrate adherence to best practices in HIV testing.
Steps to Complete the HIV Point of Care Test INSTI Quality Control Log
Completing the HIV Point of Care Test INSTI Quality Control Log involves several key steps:
- Gather necessary materials, including the log form and testing equipment.
- Conduct the quality control tests as per the manufacturer's instructions.
- Record the results of each control test in the log, noting the date and any observations.
- If any results are outside the acceptable range, document the corrective actions taken.
- Ensure that all entries are signed and dated by the responsible personnel.
By following these steps, healthcare professionals can ensure accurate and reliable testing outcomes.
Legal Use of the HIV Point of Care Test INSTI Quality Control Log
The legal use of the HIV Point of Care Test INSTI Quality Control Log is governed by various regulations that ensure the integrity of HIV testing. Compliance with these regulations is essential for the log to be considered valid in legal contexts. The log must accurately reflect quality control measures and be maintained in a manner that allows for easy access during audits or inspections. Adhering to legal requirements not only protects the testing facility but also supports public health initiatives by ensuring reliable testing results.
Key Elements of the HIV Point of Care Test INSTI Quality Control Log
Essential elements of the HIV Point of Care Test INSTI Quality Control Log include:
- Date of testing: The specific date when the quality control tests were conducted.
- Control test results: Outcomes of the quality control tests, indicating whether they are within acceptable limits.
- Corrective actions: Any actions taken in response to unacceptable results, ensuring accountability.
- Signature of personnel: Verification by the responsible individual, affirming the accuracy of the log entries.
These elements are vital for maintaining the log's integrity and ensuring compliance with regulatory standards.
How to Obtain the HIV Point of Care Test INSTI Quality Control Log
Obtaining the HIV Point of Care Test INSTI Quality Control Log is typically straightforward. Healthcare facilities can access the log through the manufacturer's resources or their internal quality assurance department. It is essential to ensure that the log is the most current version, as updates may occur to reflect changes in testing protocols or regulatory requirements. Facilities should also maintain a supply of logs to ensure uninterrupted quality control documentation.
Examples of Using the HIV Point of Care Test INSTI Quality Control Log
Using the HIV Point of Care Test INSTI Quality Control Log can vary based on specific testing scenarios. For instance:
- A clinic may use the log to document daily quality control checks, ensuring that all tests performed meet regulatory standards.
- A laboratory might utilize the log during an internal audit to verify compliance with testing protocols.
- Healthcare providers may reference the log during training sessions to illustrate the importance of quality control in HIV testing.
These examples highlight the log's role in promoting accuracy and reliability in HIV testing practices.
Quick guide on how to complete hiv point of care test insti quality control log
Prepare HIV Point Of Care Test INSTI Quality Control Log effortlessly on any device
Web-based document management has gained traction among organizations and individuals. It offers an ideal environment-friendly substitute for traditional printed and signed paperwork, allowing you to locate the appropriate form and securely store it online. airSlate SignNow provides all the resources you require to create, modify, and eSign your documents quickly without delays. Handle HIV Point Of Care Test INSTI Quality Control Log on any device with airSlate SignNow Android or iOS applications and simplify any document-related task today.
The simplest method to alter and eSign HIV Point Of Care Test INSTI Quality Control Log with ease
- Locate HIV Point Of Care Test INSTI Quality Control Log and click Get Form to begin.
- Utilize the tools we offer to complete your form.
- Emphasize important sections of your documents or obscure sensitive information with tools that airSlate SignNow provides specifically for that purpose.
- Create your eSignature using the Sign feature, which takes mere seconds and carries the same legal validity as a conventional wet ink signature.
- Review the details and click the Done button to retain your modifications.
- Choose how you wish to send your form, whether by email, text message (SMS), or invitation link, or download it to your computer.
Eliminate worries about lost or misplaced documents, tedious form-finding, or errors that necessitate printing new document copies. airSlate SignNow meets all your document management needs in just a few clicks from any device of your choosing. Modify and eSign HIV Point Of Care Test INSTI Quality Control Log and guarantee excellent communication at any stage of the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the hiv point of care test insti quality control log
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is a rapid HIV test form?
A rapid HIV test form is a document used to facilitate quick and efficient HIV testing. It allows healthcare providers to collect necessary information and consent from patients before conducting the test. This form is essential for ensuring compliance and streamlining the testing process.
-
How can I access a rapid HIV test form?
You can easily access a rapid HIV test form through our platform, airSlate SignNow. Simply sign up for our service, and you’ll be able to create, customize, and download the rapid HIV test form as needed. This ensures that you can provide testing services with the appropriate documentation.
-
What features does the rapid HIV test form offer?
The rapid HIV test form from airSlate SignNow includes features such as electronic signatures, customizable fields, and secure storage options. These features enhance the usability and efficiency of the form while maintaining accuracy and confidentiality for patient data. It’s designed to support healthcare providers in their testing processes.
-
Is the rapid HIV test form compliant with healthcare regulations?
Yes, the rapid HIV test form is designed to meet all necessary healthcare regulations and compliance standards. By using our service, you ensure that all documentation adheres to local laws and best practices regarding patient consent and data protection. This peace of mind is crucial for healthcare providers offering testing services.
-
What are the benefits of using the airSlate SignNow rapid HIV test form?
Using the airSlate SignNow rapid HIV test form offers numerous benefits, including improved efficiency in document handling and patient management. Our solution allows for quick eSigning and real-time updates, which signNowly reduces the turnaround time for processing. Additionally, it enhances the patient experience by simplifying the testing process.
-
How much does the rapid HIV test form cost?
The cost of using the rapid HIV test form on airSlate SignNow varies based on the specific plan you choose. We offer competitive pricing tailored for businesses, ensuring that even small healthcare practices can afford our services. Check our pricing page for detailed information and to find a plan that suits your needs.
-
Can I integrate the rapid HIV test form with other software?
Absolutely! The rapid HIV test form can be seamlessly integrated with various healthcare management software and applications. This integration capability enhances workflow efficiency and allows for easy data exchange, helping your practice maintain accurate records and streamline operations.
Get more for HIV Point Of Care Test INSTI Quality Control Log
Find out other HIV Point Of Care Test INSTI Quality Control Log
- How Do I eSignature North Carolina Construction LLC Operating Agreement
- eSignature Arkansas Doctors LLC Operating Agreement Later
- eSignature Tennessee Construction Contract Safe
- eSignature West Virginia Construction Lease Agreement Myself
- How To eSignature Alabama Education POA
- How To eSignature California Education Separation Agreement
- eSignature Arizona Education POA Simple
- eSignature Idaho Education Lease Termination Letter Secure
- eSignature Colorado Doctors Business Letter Template Now
- eSignature Iowa Education Last Will And Testament Computer
- How To eSignature Iowa Doctors Business Letter Template
- Help Me With eSignature Indiana Doctors Notice To Quit
- eSignature Ohio Education Purchase Order Template Easy
- eSignature South Dakota Education Confidentiality Agreement Later
- eSignature South Carolina Education Executive Summary Template Easy
- eSignature Michigan Doctors Living Will Simple
- How Do I eSignature Michigan Doctors LLC Operating Agreement
- How To eSignature Vermont Education Residential Lease Agreement
- eSignature Alabama Finance & Tax Accounting Quitclaim Deed Easy
- eSignature West Virginia Education Quitclaim Deed Fast