3654 2014-2026


What is the 3654
The FDA Form 3654, also known as the FDA standards report, is a crucial document used in the regulatory process for various products, particularly in the food and drug sectors. This form is essential for businesses seeking to demonstrate compliance with FDA regulations. The 3654 collects data related to product specifications, manufacturing practices, and safety measures. Understanding the purpose of this form is vital for companies aiming to maintain compliance and ensure the safety and efficacy of their products.
How to use the 3654
Using the FDA Form 3654 involves several steps to ensure accurate completion and submission. First, businesses must gather all necessary information regarding their product, including details about ingredients, manufacturing processes, and labeling. Next, the form should be filled out carefully, ensuring that all required fields are completed accurately. After completing the form, it is important to review it for any errors or omissions before submission. Finally, the completed form can be submitted electronically or via mail, depending on the specific requirements set by the FDA.
Steps to complete the 3654
Completing the FDA Form 3654 requires a systematic approach to ensure compliance with FDA standards. Follow these steps:
- Gather all relevant product information, including specifications and safety data.
- Access the latest version of the 3654 form, ensuring it is up to date.
- Fill out the form, paying close attention to required fields and instructions.
- Review the form for accuracy, checking for any missing information.
- Submit the form according to the FDA's specified methods, either electronically or by mail.
Legal use of the 3654
The legal use of the FDA Form 3654 is essential for compliance with federal regulations. Submitting this form accurately is a legal requirement for businesses that manufacture or distribute products regulated by the FDA. Failure to comply with these requirements can result in penalties, including fines or product recalls. Therefore, it is crucial for businesses to understand the legal implications of the information provided on the form and ensure that all data is truthful and complete.
Key elements of the 3654
The FDA Form 3654 consists of several key elements that must be included to ensure its validity. These elements typically include:
- Product identification details, such as name and description.
- Manufacturer information, including name and address.
- Specifications related to product safety and efficacy.
- Details on manufacturing processes and quality control measures.
- Signature of the responsible individual certifying the accuracy of the information.
Form Submission Methods
Submitting the FDA Form 3654 can be done through various methods, depending on the specific requirements of the FDA. Common submission methods include:
- Electronic submission via the FDA's online portal, which provides a secure and efficient way to submit documents.
- Mailing the completed form to the appropriate FDA office, ensuring it is sent via a reliable postal service.
- In-person submission at designated FDA locations, if applicable.
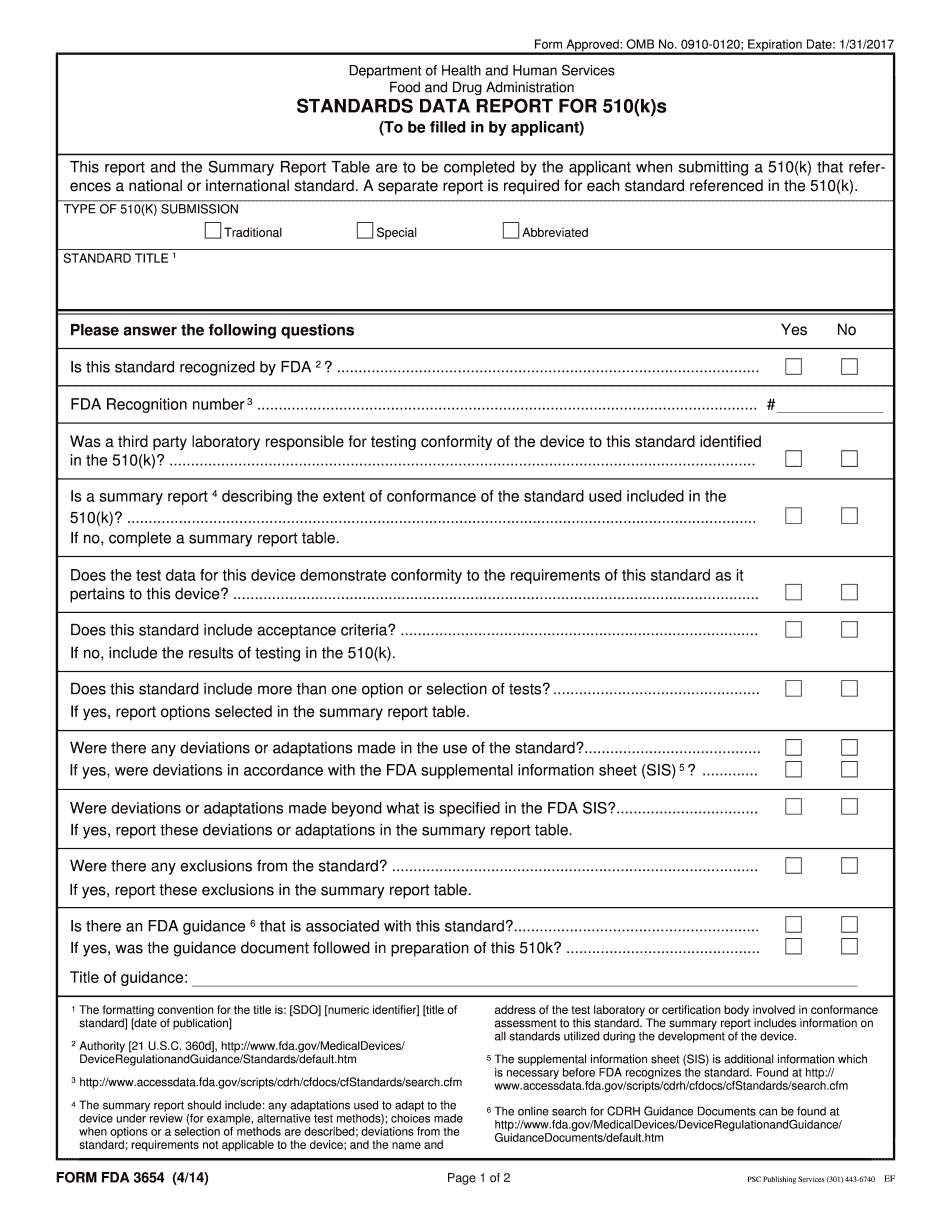
Quick guide on how to complete this report and the summary report table are to be completed by the applicant when submitting a 510k that references a national
Discover the easiest method to complete and endorse your 3654
Are you still spending time preparing your official documents on paper instead of online? airSlate SignNow offers a superior way to complete and endorse your 3654 and associated forms for public services. Our advanced eSignature solution provides you with everything necessary to handle documentation swiftly and in compliance with formal regulations - robust PDF editing, managing, protecting, endorsing, and sharing tools all available within a user-friendly interface.
You only need to take a few steps to complete and endorse your 3654:
- Load the editable template into the editor using the Get Form button.
- Review the details you need to provide in your 3654.
- Move between the fields utilizing the Next button to ensure nothing is overlooked.
- Utilize Text, Check, and Cross tools to complete the fields with your information.
- Update the content with Text boxes or Images from the upper toolbar.
- Emphasize what is important or Obscure areas that are no longer relevant.
- Press Sign to generate a legally binding eSignature using any method that suits you.
- Add the Date next to your signature and finalize your task with the Done button.
Store your finalized 3654 in the Documents section within your account, download it, or transfer it to your preferred cloud storage. Our tool also provides versatile file sharing options. There’s no need to print your forms when sending them to the appropriate public office - do it via email, fax, or by requesting a USPS “snail mail” delivery from your account. Experience it today!
Create this form in 5 minutes or less
Find and fill out the correct this report and the summary report table are to be completed by the applicant when submitting a 510k that references a national
Create this form in 5 minutes!
How to create an eSignature for the this report and the summary report table are to be completed by the applicant when submitting a 510k that references a national
How to generate an eSignature for your This Report And The Summary Report Table Are To Be Completed By The Applicant When Submitting A 510k That References A National in the online mode
How to make an eSignature for your This Report And The Summary Report Table Are To Be Completed By The Applicant When Submitting A 510k That References A National in Chrome
How to generate an eSignature for putting it on the This Report And The Summary Report Table Are To Be Completed By The Applicant When Submitting A 510k That References A National in Gmail
How to create an eSignature for the This Report And The Summary Report Table Are To Be Completed By The Applicant When Submitting A 510k That References A National straight from your mobile device
How to generate an electronic signature for the This Report And The Summary Report Table Are To Be Completed By The Applicant When Submitting A 510k That References A National on iOS devices
How to create an eSignature for the This Report And The Summary Report Table Are To Be Completed By The Applicant When Submitting A 510k That References A National on Android devices
People also ask
-
What is the significance of an FDA standards report?
The FDA standards report is crucial for businesses aiming to comply with regulations in the healthcare and pharmaceutical sectors. It outlines the specific requirements that need to be met, ensuring that your documents align with industry standards. By understanding these requirements, you can streamline your workflow and avoid potential compliance issues.
-
How does airSlate SignNow support compliance with FDA standards?
airSlate SignNow helps businesses adhere to FDA standards by providing secure and compliant eSignature solutions. Our platform maintains an audit trail and electronic records, which are essential for meeting FDA requirements. This way, you can ensure that your documentation processes are both efficient and compliant with FDA standards.
-
Are there any costs associated with obtaining an FDA standards report via airSlate SignNow?
While airSlate SignNow offers affordable eSignature solutions, obtaining FDA standards reports typically requires consultation with regulatory experts. However, the platform itself helps reduce costs by automating document management and eSigning. This efficiency can lower the overall expenses associated with compliance efforts.
-
Can airSlate SignNow integrate with other compliance tools related to FDA standards?
Yes, airSlate SignNow offers a variety of integrations with compliance tools and software that can assist in managing FDA standards. This allows for seamless data transfer and enhanced compliance tracking. By integrating with existing systems, businesses can ensure a more robust approach to compliance with FDA standards.
-
What benefits does airSlate SignNow provide for businesses needing FDA standards report compliance?
Using airSlate SignNow, businesses can efficiently manage their compliance processes related to FDA standards reports. The platform enhances productivity through easy document eSigning and provides features like templates and automated workflows. This not only speeds up the compliance process but also helps maintain the integrity of your documents.
-
How secure is the documentation process when dealing with FDA standards reports in airSlate SignNow?
airSlate SignNow prioritizes security, employing advanced encryption and secure data storage to protect sensitive information related to FDA standards reports. Our compliance with various regulations ensures that your documents remain confidential and tamper-proof. This level of security is critical for businesses navigating FDA compliance.
-
Is training available for utilizing airSlate SignNow for FDA standards report compliance?
Yes, airSlate SignNow provides comprehensive training and resources to help users understand how to handle FDA standards reports effectively. Our support team is available to guide you through the features and functionalities relevant to compliance. Proper training ensures that your team can leverage the platform to meet FDA requirements adequately.
Get more for 3654
- Online mspcindia org form
- Body treatment consultation form
- Annual earnings statement for disability benefits sers 220 annual earnings statement for disability benefits sers 220 pdf form
- Annual comprehensive diabetes foot exam form diabetes foot exam
- Certificate of tax exemption affidavit form
- Kniffel vorlage pdf form
- Prior auth for meritus medication form
- Stop 6525 sp cis kansas city mo form
Find out other 3654
- How Do I eSignature Montana Construction Claim
- eSignature Construction PPT New Jersey Later
- How Do I eSignature North Carolina Construction LLC Operating Agreement
- eSignature Arkansas Doctors LLC Operating Agreement Later
- eSignature Tennessee Construction Contract Safe
- eSignature West Virginia Construction Lease Agreement Myself
- How To eSignature Alabama Education POA
- How To eSignature California Education Separation Agreement
- eSignature Arizona Education POA Simple
- eSignature Idaho Education Lease Termination Letter Secure
- eSignature Colorado Doctors Business Letter Template Now
- eSignature Iowa Education Last Will And Testament Computer
- How To eSignature Iowa Doctors Business Letter Template
- Help Me With eSignature Indiana Doctors Notice To Quit
- eSignature Ohio Education Purchase Order Template Easy
- eSignature South Dakota Education Confidentiality Agreement Later
- eSignature South Carolina Education Executive Summary Template Easy
- eSignature Michigan Doctors Living Will Simple
- How Do I eSignature Michigan Doctors LLC Operating Agreement
- How To eSignature Vermont Education Residential Lease Agreement