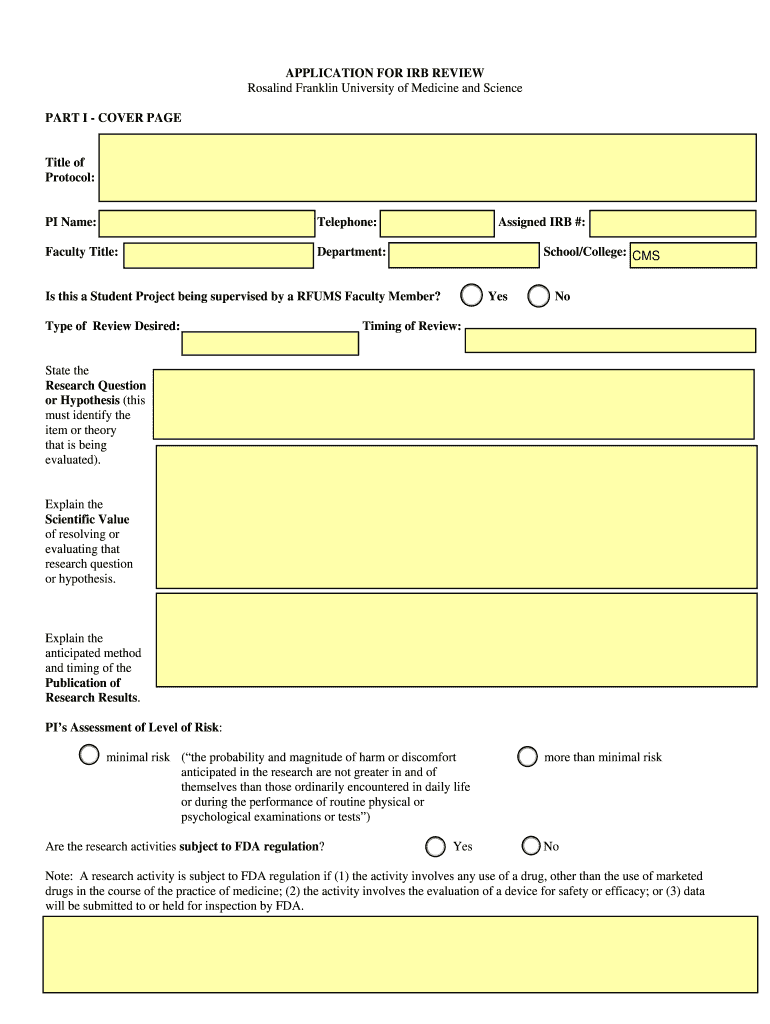
Initial, Continuing, and Modification Application for Review Form


What is the Initial, Continuing, And Modification Application For Review
The Initial, Continuing, and Modification Application for Review is a formal document used in various legal and administrative contexts to request a review of decisions or actions taken by governmental or regulatory bodies. This application is crucial for individuals or entities seeking to contest, modify, or continue a previous decision regarding their case or status. It serves as a structured means to present relevant information and arguments to support the request for review.
How to use the Initial, Continuing, And Modification Application For Review
Using the Initial, Continuing, and Modification Application for Review involves several key steps. First, gather all necessary information related to your case, including previous decisions and any supporting documentation. Next, carefully complete the application form, ensuring that all sections are filled out accurately. It is important to clearly articulate the reasons for your request, providing any evidence or arguments that support your position. Finally, submit the application according to the specified guidelines, whether online, by mail, or in person, depending on the requirements of the relevant authority.
Steps to complete the Initial, Continuing, And Modification Application For Review
Completing the Initial, Continuing, and Modification Application for Review requires attention to detail and adherence to specific guidelines. Follow these steps for a successful submission:
- Review the instructions provided with the application form to understand the requirements.
- Gather all necessary documents, including previous decisions and supporting evidence.
- Fill out the application form, ensuring all required fields are completed accurately.
- Clearly state your reasons for requesting a review, supported by relevant facts.
- Double-check your application for any errors or omissions before submission.
- Submit the application through the designated method, ensuring you keep a copy for your records.
Required Documents
When submitting the Initial, Continuing, and Modification Application for Review, certain documents are typically required to support your request. These may include:
- A copy of the original decision or action being contested.
- Any relevant correspondence related to the case.
- Supporting evidence, such as witness statements or expert opinions.
- Identification documents, if applicable.
Ensure that all documents are clear and legible to facilitate the review process.
Eligibility Criteria
Eligibility to submit the Initial, Continuing, and Modification Application for Review can vary based on the specific context or governing body. Generally, individuals or entities directly affected by a decision or action have the right to apply for a review. It is essential to check the specific eligibility criteria outlined by the relevant authority to ensure compliance and avoid delays in the review process.
Form Submission Methods
The Initial, Continuing, and Modification Application for Review can typically be submitted through various methods, depending on the requirements of the governing body. Common submission methods include:
- Online submission through a designated portal.
- Mailing the completed application to the appropriate office.
- In-person submission at a specified location.
Each method may have its own set of guidelines, including deadlines and required formats, so it is important to follow the instructions carefully.
Quick guide on how to complete initial continuing and modification application for review
Complete [SKS] effortlessly on any device
Digital document management has become increasingly favored by businesses and individuals alike. It serves as an ideal eco-friendly substitute for traditional printed and signed papers, allowing you to find the correct format and securely store it online. airSlate SignNow provides you with all the resources you require to create, modify, and eSign your documents promptly without delays. Handle [SKS] on any platform with airSlate SignNow's Android or iOS applications and simplify any document-related task today.
How to alter and eSign [SKS] with ease
- Locate [SKS] and hit Get Form to begin.
- Make use of the tools we provide to fill out your document.
- Emphasize relevant sections of the documents or obscure sensitive details with instruments that airSlate SignNow specifically offers for that purpose.
- Generate your eSignature using the Sign tool, which takes mere seconds and has the same legal validity as a conventional wet ink signature.
- Verify the information and click on the Done button to preserve your changes.
- Select how you wish to send your form, whether by email, text message (SMS), or invite link, or download it to your computer.
Put an end to missing or lost documents, the hassle of searching for forms, or mistakes that require printing new document copies. airSlate SignNow meets your document management needs in just a few clicks from any device you prefer. Edit and eSign [SKS] and ensure effective communication at every stage of the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the initial continuing and modification application for review
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is the Initial, Continuing, And Modification Application For Review process?
The Initial, Continuing, And Modification Application For Review process involves submitting necessary documentation to ensure compliance with regulatory standards. This process is crucial for businesses looking to maintain their operational licenses and certifications. airSlate SignNow simplifies this process by providing a platform for easy document management and eSigning.
-
How can airSlate SignNow help with the Initial, Continuing, And Modification Application For Review?
airSlate SignNow streamlines the Initial, Continuing, And Modification Application For Review by allowing users to create, send, and sign documents electronically. This reduces the time spent on paperwork and enhances collaboration among stakeholders. With our user-friendly interface, you can manage your applications efficiently.
-
What are the pricing options for using airSlate SignNow for my Initial, Continuing, And Modification Application For Review?
airSlate SignNow offers flexible pricing plans tailored to meet the needs of businesses of all sizes. Whether you are a small startup or a large enterprise, you can find a plan that fits your budget while ensuring you have the tools necessary for your Initial, Continuing, And Modification Application For Review. Visit our pricing page for detailed information.
-
Are there any features specifically designed for the Initial, Continuing, And Modification Application For Review?
Yes, airSlate SignNow includes features such as customizable templates, automated workflows, and secure cloud storage that are ideal for the Initial, Continuing, And Modification Application For Review. These features help ensure that your documents are compliant and easily accessible. Additionally, our platform supports real-time collaboration, making it easier to gather necessary approvals.
-
What benefits does airSlate SignNow provide for managing the Initial, Continuing, And Modification Application For Review?
Using airSlate SignNow for your Initial, Continuing, And Modification Application For Review offers numerous benefits, including increased efficiency, reduced errors, and enhanced security. Our electronic signature solution ensures that your documents are signed quickly and securely, while our tracking features allow you to monitor the status of your applications in real-time.
-
Can I integrate airSlate SignNow with other tools for my Initial, Continuing, And Modification Application For Review?
Absolutely! airSlate SignNow integrates seamlessly with various third-party applications, enhancing your workflow for the Initial, Continuing, And Modification Application For Review. Whether you use CRM systems, project management tools, or cloud storage services, our integrations help you maintain a cohesive and efficient process.
-
Is airSlate SignNow compliant with regulations for the Initial, Continuing, And Modification Application For Review?
Yes, airSlate SignNow is designed to comply with industry regulations, ensuring that your Initial, Continuing, And Modification Application For Review meets all necessary legal standards. Our platform adheres to eSignature laws and data protection regulations, providing peace of mind as you manage your documents.
Get more for Initial, Continuing, And Modification Application For Review
- Get the free backflow prevention assembly certified test form
- Bringing off exchange consumers to covered california form
- 2020 form eden press the original privacy catalog fill
- Spelman college office of the registrar350 spelman lane s form
- American equity partial withdrawal request form
- Mdec counties only unless you are filing into a restricted case type adoption emergency evaluation form
- Ucmc 218 form
- Transcript information office of the registrar baylor
Find out other Initial, Continuing, And Modification Application For Review
- Electronic signature Finance & Tax Accounting Word South Carolina Later
- How Do I Electronic signature Illinois Healthcare / Medical Purchase Order Template
- Electronic signature Louisiana Healthcare / Medical Quitclaim Deed Online
- Electronic signature Louisiana Healthcare / Medical Quitclaim Deed Computer
- How Do I Electronic signature Louisiana Healthcare / Medical Limited Power Of Attorney
- Electronic signature Maine Healthcare / Medical Letter Of Intent Fast
- How To Electronic signature Mississippi Healthcare / Medical Month To Month Lease
- Electronic signature Nebraska Healthcare / Medical RFP Secure
- Electronic signature Nevada Healthcare / Medical Emergency Contact Form Later
- Electronic signature New Hampshire Healthcare / Medical Credit Memo Easy
- Electronic signature New Hampshire Healthcare / Medical Lease Agreement Form Free
- Electronic signature North Dakota Healthcare / Medical Notice To Quit Secure
- Help Me With Electronic signature Ohio Healthcare / Medical Moving Checklist
- Electronic signature Education PPT Ohio Secure
- Electronic signature Tennessee Healthcare / Medical NDA Now
- Electronic signature Tennessee Healthcare / Medical Lease Termination Letter Online
- Electronic signature Oklahoma Education LLC Operating Agreement Fast
- How To Electronic signature Virginia Healthcare / Medical Contract
- How To Electronic signature Virginia Healthcare / Medical Operating Agreement
- Electronic signature Wisconsin Healthcare / Medical Business Letter Template Mobile