Annex 1 Clinical Trial Application Form the Europa Eu


What is the Annex 1 Clinical Trial Application Form The Europa eu
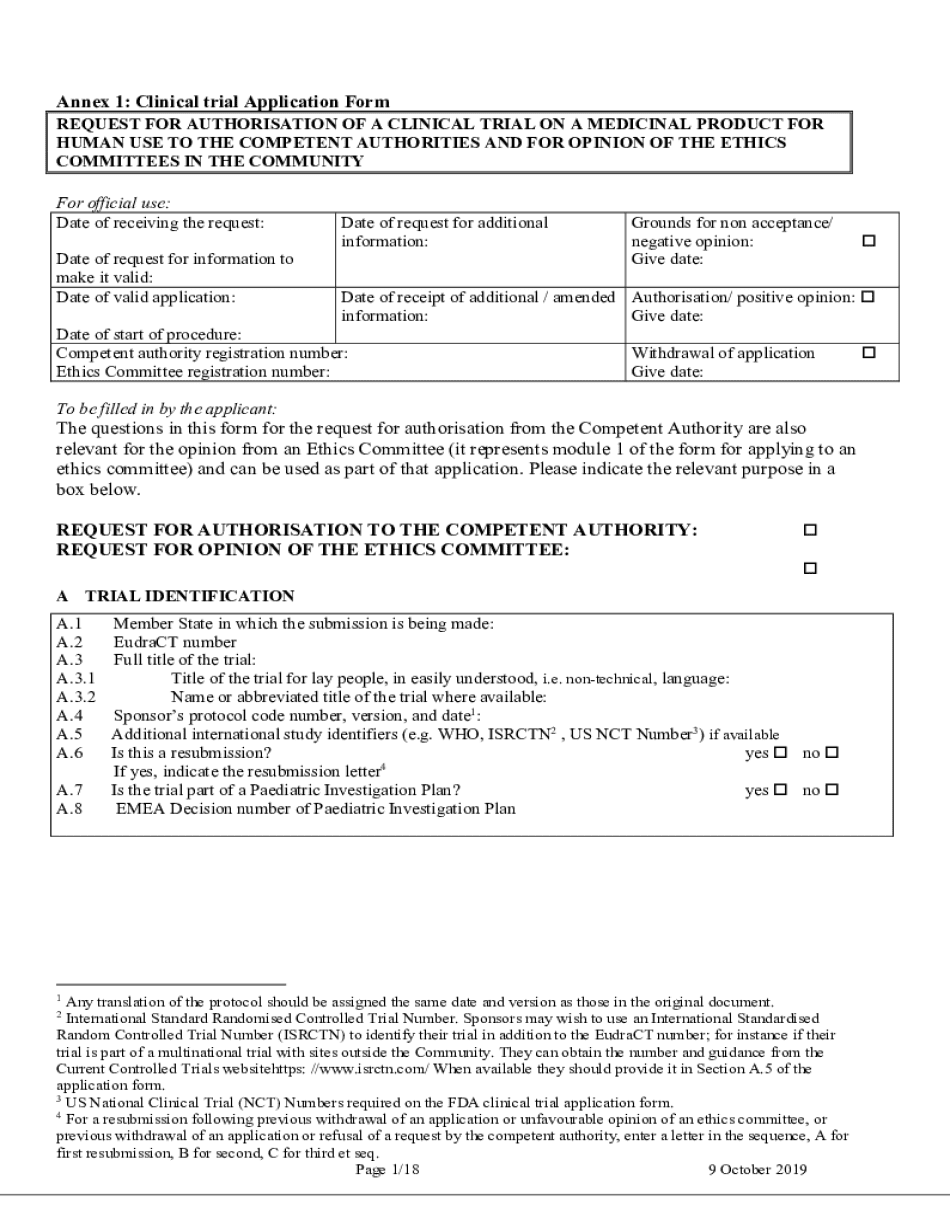
The Annex 1 Clinical Trial Application Form The Europa eu is a crucial document used in the clinical trial approval process within the European Union. This form is designed to collect essential information about the clinical trial, including the study's objectives, methodology, and the qualifications of the research team. It ensures that all necessary data is available for regulatory authorities to assess the trial's compliance with safety and ethical standards.
Key elements of the Annex 1 Clinical Trial Application Form The Europa eu
Understanding the key elements of the Annex 1 Clinical Trial Application Form is vital for successful completion. The form typically includes sections for:
- Trial Identification: Basic information such as the trial title, identifier, and sponsor details.
- Study Objectives: Clear articulation of the primary and secondary objectives of the trial.
- Methodology: Detailed description of the study design, including participant selection and intervention methods.
- Ethical Considerations: Information on how the trial addresses ethical issues and participant safety.
- Data Management: Plans for data collection, storage, and analysis, ensuring compliance with data protection regulations.
Steps to complete the Annex 1 Clinical Trial Application Form The Europa eu
Completing the Annex 1 Clinical Trial Application Form involves several critical steps:
- Gather Information: Collect all necessary data regarding the trial, including objectives, methodology, and ethical considerations.
- Fill Out the Form: Carefully complete each section of the form, ensuring accuracy and clarity in your responses.
- Review and Edit: Thoroughly review the completed form for any errors or omissions. It may be beneficial to have a colleague provide feedback.
- Submit the Form: Follow the designated submission process, ensuring that all required documents are included.
How to obtain the Annex 1 Clinical Trial Application Form The Europa eu
The Annex 1 Clinical Trial Application Form can typically be obtained from regulatory authorities or official government websites related to clinical trials. In the United States, researchers may need to consult the FDA or other relevant bodies for specific guidelines on accessing and completing the form. It is essential to ensure that you are using the most current version of the form to comply with regulatory standards.
Legal use of the Annex 1 Clinical Trial Application Form The Europa eu
The legal use of the Annex 1 Clinical Trial Application Form is governed by various regulations that ensure the protection of human subjects and the integrity of clinical trials. Researchers must adhere to the guidelines set forth by regulatory authorities, including obtaining necessary approvals before initiating the trial. Failure to comply with these regulations can result in penalties, including fines or disqualification from conducting future research.
Form Submission Methods (Online / Mail / In-Person)
Submitting the Annex 1 Clinical Trial Application Form can be done through various methods, depending on the regulatory requirements. Common submission methods include:
- Online Submission: Many regulatory bodies offer online platforms for submitting forms, which can streamline the process and reduce paperwork.
- Mail Submission: Researchers may also submit the form via traditional mail, ensuring that all documents are securely packaged and sent to the correct address.
- In-Person Submission: In some cases, submitting the form in person may be required, particularly for sensitive or complex applications.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the annex 1 clinical trial application form the europa eu
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is the Annex 1 Clinical Trial Application Form The Europa eu?
The Annex 1 Clinical Trial Application Form The Europa eu is a standardized document required for submitting clinical trial applications in Europe. It ensures that all necessary information is provided to regulatory authorities for review. Understanding this form is crucial for compliance and successful trial initiation.
-
How can airSlate SignNow help with the Annex 1 Clinical Trial Application Form The Europa eu?
airSlate SignNow simplifies the process of completing and submitting the Annex 1 Clinical Trial Application Form The Europa eu. Our platform allows users to easily fill out, sign, and send documents securely. This streamlines the application process, ensuring timely submissions and compliance.
-
What are the pricing options for using airSlate SignNow for the Annex 1 Clinical Trial Application Form The Europa eu?
airSlate SignNow offers flexible pricing plans tailored to meet the needs of businesses handling the Annex 1 Clinical Trial Application Form The Europa eu. Our plans are designed to be cost-effective, providing access to essential features without breaking the budget. Contact us for a detailed quote based on your specific requirements.
-
What features does airSlate SignNow offer for managing the Annex 1 Clinical Trial Application Form The Europa eu?
Our platform includes features such as customizable templates, electronic signatures, and secure document storage specifically for the Annex 1 Clinical Trial Application Form The Europa eu. These tools enhance efficiency and ensure that all documents are compliant with regulatory standards. Additionally, our user-friendly interface makes it easy for teams to collaborate.
-
Are there any integrations available with airSlate SignNow for the Annex 1 Clinical Trial Application Form The Europa eu?
Yes, airSlate SignNow integrates seamlessly with various applications to enhance the management of the Annex 1 Clinical Trial Application Form The Europa eu. This includes popular tools for project management, CRM, and document storage. These integrations help streamline workflows and improve overall productivity.
-
What are the benefits of using airSlate SignNow for the Annex 1 Clinical Trial Application Form The Europa eu?
Using airSlate SignNow for the Annex 1 Clinical Trial Application Form The Europa eu offers numerous benefits, including increased efficiency, reduced processing time, and enhanced compliance. Our platform ensures that all documents are securely signed and stored, minimizing the risk of errors. This allows teams to focus on what matters most—conducting successful clinical trials.
-
Is airSlate SignNow compliant with regulations for the Annex 1 Clinical Trial Application Form The Europa eu?
Absolutely, airSlate SignNow is designed to comply with all relevant regulations for the Annex 1 Clinical Trial Application Form The Europa eu. We prioritize data security and legal compliance, ensuring that your documents meet all necessary standards. This gives users peace of mind when managing sensitive clinical trial information.
Get more for Annex 1 Clinical Trial Application Form The Europa eu
- 19 printable custom t shirt order form templates
- Constructing a losers bracket in a seeded double form
- Well do thistogethergreen pet burial society form
- Bis 711 guidance form
- Stock certificate announcement form
- Pick up player form usa softball
- Gegevens betaling buitenland non sepa form
- Denna blankett anvnds fr bouppteckning med anledning av form
Find out other Annex 1 Clinical Trial Application Form The Europa eu
- Help Me With Sign Nebraska Business Operations Presentation
- How To Sign Arizona Car Dealer Form
- How To Sign Arkansas Car Dealer Document
- How Do I Sign Colorado Car Dealer PPT
- Can I Sign Florida Car Dealer PPT
- Help Me With Sign Illinois Car Dealer Presentation
- How Can I Sign Alabama Charity Form
- How Can I Sign Idaho Charity Presentation
- How Do I Sign Nebraska Charity Form
- Help Me With Sign Nevada Charity PDF
- How To Sign North Carolina Charity PPT
- Help Me With Sign Ohio Charity Document
- How To Sign Alabama Construction PDF
- How To Sign Connecticut Construction Document
- How To Sign Iowa Construction Presentation
- How To Sign Arkansas Doctors Document
- How Do I Sign Florida Doctors Word
- Can I Sign Florida Doctors Word
- How Can I Sign Illinois Doctors PPT
- How To Sign Texas Doctors PDF