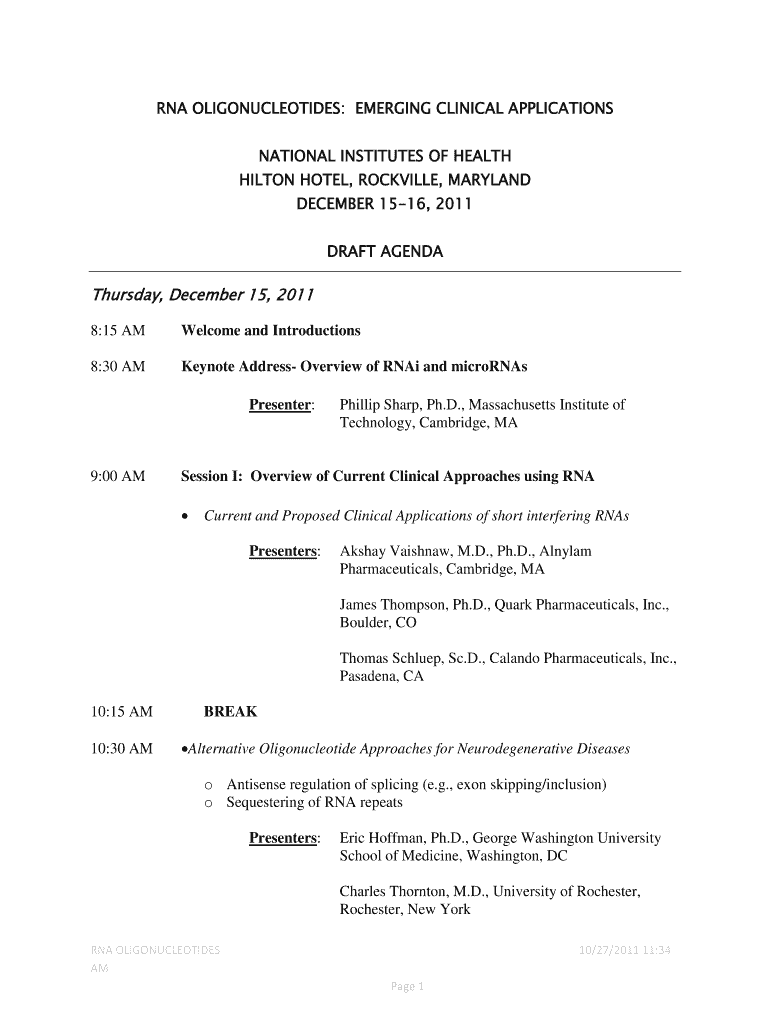
Clinical Applications of Oligonucleotides Form


What is the clinical applications of oligonucleotides form
The clinical applications of oligonucleotides form is a specialized document used in the medical and research fields to outline the use of oligonucleotides in various clinical settings. Oligonucleotides, which are short sequences of nucleotides, play a crucial role in genetic testing, therapeutic interventions, and diagnostic procedures. This form captures essential information about the intended use, patient details, and regulatory compliance necessary for the application of oligonucleotides in clinical practice.
How to use the clinical applications of oligonucleotides form
Using the clinical applications of oligonucleotides form involves several key steps. First, gather all relevant patient information, including medical history and specific details about the oligonucleotide treatment or testing. Next, ensure that the form is filled out completely and accurately, as incomplete information may lead to delays or complications in processing. It is important to review the form for any specific instructions related to the clinical application, as these can vary based on the institution or regulatory body overseeing the use of oligonucleotides.
Key elements of the clinical applications of oligonucleotides form
Several key elements must be included in the clinical applications of oligonucleotides form to ensure its validity and compliance with regulatory standards. These elements typically include:
- Patient identification: Full name, date of birth, and contact information.
- Clinical indication: A clear description of the reason for using oligonucleotides, including any relevant medical conditions.
- Type of oligonucleotide: Specification of the oligonucleotide sequence or product being used.
- Provider information: Details about the healthcare provider or institution responsible for the application.
- Consent: Documentation of informed consent from the patient or their legal representative.
Steps to complete the clinical applications of oligonucleotides form
Completing the clinical applications of oligonucleotides form involves a systematic approach to ensure accuracy and compliance. Follow these steps:
- Collect all necessary patient and clinical information.
- Fill out the form, ensuring each section is completed as per the guidelines.
- Review the form for accuracy, checking for any missing information or errors.
- Obtain the required signatures and consents.
- Submit the completed form according to the specified submission methods.
Legal use of the clinical applications of oligonucleotides form
The legal use of the clinical applications of oligonucleotides form is governed by various regulations that ensure the protection of patient rights and the integrity of medical practices. Compliance with federal and state laws, including HIPAA for patient privacy, is essential. Additionally, the form must adhere to guidelines set forth by regulatory bodies overseeing genetic testing and therapeutic applications. Proper documentation and consent are crucial to mitigate legal risks associated with the use of oligonucleotides in clinical settings.
Examples of using the clinical applications of oligonucleotides form
There are various scenarios in which the clinical applications of oligonucleotides form is utilized. Examples include:
- Genetic testing: Used to obtain consent for testing patients for hereditary conditions.
- Therapeutic applications: Documenting the use of oligonucleotides in targeted therapies for conditions like cancer.
- Research studies: Required for enrolling participants in clinical trials involving oligonucleotide treatments.
Quick guide on how to complete clinical applications of oligonucleotides form
Effortlessly Prepare Clinical Applications Of Oligonucleotides Form on Any Device
Digital document management has become favored by both companies and individuals. It serves as an ideal environmentally friendly alternative to traditional printed and signed documents, allowing you to obtain the correct form and securely store it online. airSlate SignNow offers you all the tools necessary to create, modify, and electronically sign your documents swiftly without any holdups. Handle Clinical Applications Of Oligonucleotides Form on any device using the airSlate SignNow Android or iOS applications and streamline your document processes today.
The simplest way to modify and electronically sign Clinical Applications Of Oligonucleotides Form with ease
- Obtain Clinical Applications Of Oligonucleotides Form and click on Get Form to begin.
- Utilize the tools we offer to complete your document.
- Emphasize essential sections of your documents or obscure confidential information with tools that airSlate SignNow specifically offers for that reason.
- Generate your signature using the Sign feature, which only takes seconds and holds the same legal validity as a conventional wet ink signature.
- Review all details and click on the Done button to retain your changes.
- Select your preferred method to send your form, whether by email, SMS, or invitation link, or download it to your computer.
Eliminate concerns about lost or misplaced documents, tedious form searches, or mistakes that require printing additional document copies. airSlate SignNow fulfills all your document management needs with just a few clicks from any device you select. Edit and electronically sign Clinical Applications Of Oligonucleotides Form while ensuring excellent communication throughout the document preparation process with airSlate SignNow.
Create this form in 5 minutes or less
FAQs
-
How can I fill out Google's intern host matching form to optimize my chances of receiving a match?
I was selected for a summer internship 2016.I tried to be very open while filling the preference form: I choose many products as my favorite products and I said I'm open about the team I want to join.I even was very open in the location and start date to get host matching interviews (I negotiated the start date in the interview until both me and my host were happy.) You could ask your recruiter to review your form (there are very cool and could help you a lot since they have a bigger experience).Do a search on the potential team.Before the interviews, try to find smart question that you are going to ask for the potential host (do a search on the team to find nice and deep questions to impress your host). Prepare well your resume.You are very likely not going to get algorithm/data structure questions like in the first round. It's going to be just some friendly chat if you are lucky. If your potential team is working on something like machine learning, expect that they are going to ask you questions about machine learning, courses related to machine learning you have and relevant experience (projects, internship). Of course you have to study that before the interview. Take as long time as you need if you feel rusty. It takes some time to get ready for the host matching (it's less than the technical interview) but it's worth it of course.
-
How do I fill out the form of DU CIC? I couldn't find the link to fill out the form.
Just register on the admission portal and during registration you will get an option for the entrance based course. Just register there. There is no separate form for DU CIC.
-
How can I fill out the online application form of JVM Shyamli Ranchi?
Go to Jawahar Vidiya Mandir website
-
How do I fill out an application form to open a bank account?
I want to believe that most banks nowadays have made the process of opening bank account, which used to be cumbersome, less cumbersome. All you need to do is to approach the bank, collect the form, and fill. However if you have any difficulty in filling it, you can always call on one of the banks rep to help you out.
-
What is the last date to fill out the application form of the Indian Navy?
Hello VinayThe last date for filling the form has already gone. It was March 4, 2018. Kindly wait for the next application date to come.RegardsAnkita
Create this form in 5 minutes!
How to create an eSignature for the clinical applications of oligonucleotides form
How to make an electronic signature for the Clinical Applications Of Oligonucleotides Form in the online mode
How to generate an electronic signature for your Clinical Applications Of Oligonucleotides Form in Google Chrome
How to make an electronic signature for putting it on the Clinical Applications Of Oligonucleotides Form in Gmail
How to create an eSignature for the Clinical Applications Of Oligonucleotides Form straight from your mobile device
How to generate an eSignature for the Clinical Applications Of Oligonucleotides Form on iOS
How to create an electronic signature for the Clinical Applications Of Oligonucleotides Form on Android
People also ask
-
What are the clinical applications of oligonucleotides?
The clinical applications of oligonucleotides are vast, ranging from diagnostics to therapeutics. These small DNA or RNA molecules can be designed to target specific genes, making them effective in gene therapy and precision medicine. Their versatility allows them to play essential roles in treating genetic disorders, cancer, and infectious diseases.
-
How can airSlate SignNow support the documentation for clinical applications of oligonucleotides?
airSlate SignNow streamlines the documentation process by enabling the easy eSigning and sharing of critical documents related to the clinical application of oligonucleotides. With features that ensure compliance and security, this solution helps researchers and healthcare professionals efficiently manage consent forms and regulatory submissions.
-
Are there any costs associated with using airSlate SignNow for clinical applications of oligonucleotides?
Yes, there are costs associated with using airSlate SignNow, but it offers a cost-effective solution for managing documents related to clinical applications of oligonucleotides. Pricing plans vary based on features and the number of users, allowing businesses to choose a plan that suits their specific needs without compromising on quality.
-
What features does airSlate SignNow provide for managing clinical documentation?
airSlate SignNow offers a variety of features, including customizable templates, secure cloud storage, and tracking tools, to facilitate the management of clinical documentation related to oligonucleotides. These features improve collaboration and ensure that all parties can access the necessary documents readily, ultimately enhancing the clinical research process.
-
Can airSlate SignNow integrate with other tools relevant to clinical applications of oligonucleotides?
Absolutely, airSlate SignNow can integrate seamlessly with various software and tools commonly used in clinical research. This integration ability allows researchers working on the clinical application of oligonucleotides to maintain workflow efficiency, ensuring that document management is synchronized with other research and healthcare systems.
-
What are the benefits of using airSlate SignNow for clinical research?
Using airSlate SignNow for clinical research offers numerous benefits, including increased efficiency, compliance, and cost savings. It simplifies the signing and management of documentation, which is crucial for the clinical application of oligonucleotides, ensuring that researchers and practitioners can focus more on their work rather than paperwork.
-
How does airSlate SignNow ensure the security of documents in clinical applications?
airSlate SignNow prioritizes document security with advanced encryption and authentication measures to protect sensitive information, which is especially important in clinical applications of oligonucleotides. This ensures that all electronic signatures and documents comply with industry standards and regulations, safeguarding against unauthorized access.
Get more for Clinical Applications Of Oligonucleotides Form
- La paf 0658 outpatient prior authorization form outpatient prior authorization form
- Form la paf 0658
- Loss damage waiver premier bautob credit form
- Rental car loss damage waiver cdw insuranceavis rent form
- Correction application form
- Tus digital badge earner application and consent form
- Debeka formulare zum ausdrucken
- Novo nordisk patient assistance program form
Find out other Clinical Applications Of Oligonucleotides Form
- Can I eSignature Texas New hire forms
- How Can I eSignature California New hire packet
- How To eSignature South Carolina Real estate document
- eSignature Florida Real estate investment proposal template Free
- How To eSignature Utah Real estate forms
- How Do I eSignature Washington Real estate investment proposal template
- Can I eSignature Kentucky Performance Contract
- eSignature Nevada Performance Contract Safe
- eSignature California Franchise Contract Secure
- How To eSignature Colorado Sponsorship Proposal Template
- eSignature Alabama Distributor Agreement Template Secure
- eSignature California Distributor Agreement Template Later
- eSignature Vermont General Power of Attorney Template Easy
- eSignature Michigan Startup Cost Estimate Simple
- eSignature New Hampshire Invoice for Services (Standard Format) Computer
- eSignature Arkansas Non-Compete Agreement Later
- Can I eSignature Arizona Non-Compete Agreement
- How Do I eSignature New Jersey Non-Compete Agreement
- eSignature Tennessee Non-Compete Agreement Myself
- How To eSignature Colorado LLC Operating Agreement