Fda 2541a Form


What is the FDA 2541a?
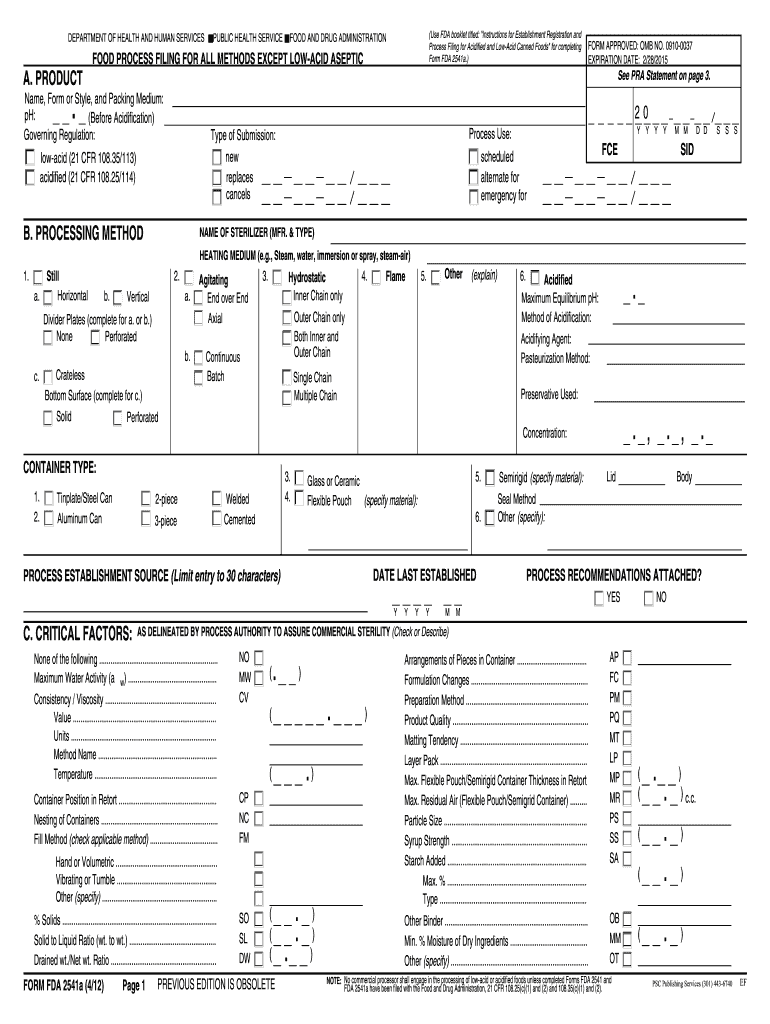
The FDA 2541a is a critical form used in the food industry, particularly for the submission of information related to food processing methods. This form is essential for manufacturers and processors to demonstrate compliance with safety regulations set forth by the Food and Drug Administration (FDA). It specifically pertains to low-acid and acidified foods, ensuring that these products meet safety standards to protect consumers.
Steps to Complete the FDA 2541a
Completing the FDA 2541a requires careful attention to detail. Here are the essential steps:
- Gather necessary information about the food product, including its composition and processing methods.
- Ensure that all safety protocols are in place during the preparation of the form.
- Fill out the form accurately, providing all required details regarding the food process.
- Review the completed form for any errors or omissions before submission.
- Submit the form to the appropriate FDA office, either online or by mail.
Legal Use of the FDA 2541a
The legal use of the FDA 2541a is governed by various regulations that ensure food safety. Compliance with these regulations is crucial for manufacturers to avoid penalties. The form serves as a declaration that the food product adheres to safety standards, and it must be filled out truthfully to maintain legal protection. Misrepresentation on the form can lead to serious legal consequences.
Key Elements of the FDA 2541a
Understanding the key elements of the FDA 2541a is vital for successful completion. The form typically includes:
- Product identification details, including name and type of food.
- Processing methods used, such as heating or acidification.
- Information on the ingredients and their sources.
- Details on packaging and labeling requirements.
Form Submission Methods
The FDA 2541a can be submitted through various methods, offering flexibility to users. The primary submission methods include:
- Online submission through the FDA's designated portal.
- Mailing a hard copy of the completed form to the appropriate FDA office.
- In-person submission at FDA regional offices, if necessary.
Examples of Using the FDA 2541a
Examples of using the FDA 2541a can help clarify its application. Common scenarios include:
- Manufacturers of low-acid canned foods must submit the form to ensure compliance with safety standards.
- Producers of acidified foods, such as pickles or sauces, utilize the form to document their processing methods.
- Food processors seeking to market new products may need to complete the form to gain FDA approval.
Quick guide on how to complete fda 2541a
Complete Fda 2541a effortlessly on any device
Digital document management has become increasingly favored by organizations and individuals alike. It offers an ideal eco-conscious alternative to conventional printed and signed papers, allowing you to locate the right template and securely preserve it online. airSlate SignNow equips you with all the necessary tools to create, modify, and eSign your documents quickly and without complications. Manage Fda 2541a on any device using airSlate SignNow's Android or iOS applications and streamline your document-related tasks today.
How to modify and eSign Fda 2541a with ease
- Locate Fda 2541a and then click Get Form to begin.
- Utilize the tools we offer to fill out your form.
- Select important sections of your documents or obscure sensitive data with tools specifically designed by airSlate SignNow for that purpose.
- Generate your eSignature with the Sign tool, which takes only seconds and holds the same legal validity as a conventional wet ink signature.
- Review the details and then click on the Done button to save your modifications.
- Choose your preferred method to send your form: via email, text message (SMS), or invitation link, or download it to your computer.
Eliminate concerns about lost or misplaced documents, tedious form searching, or errors requiring new document copies to be printed. airSlate SignNow addresses all your document management needs in just a few clicks from your chosen device. Alter and eSign Fda 2541a, ensuring excellent communication throughout the document preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the fda 2541a
The way to make an electronic signature for a PDF document in the online mode
The way to make an electronic signature for a PDF document in Chrome
The best way to generate an eSignature for putting it on PDFs in Gmail
How to generate an electronic signature straight from your mobile device
The way to generate an eSignature for a PDF document on iOS devices
How to generate an electronic signature for a PDF document on Android devices
People also ask
-
What is FDA safety, and how does it relate to airSlate SignNow?
FDA safety refers to the regulations and guidelines set by the Food and Drug Administration to ensure the safety and efficacy of products, including digital solutions like airSlate SignNow. By utilizing airSlate SignNow, businesses can maintain compliance with these standards, ensuring that all electronic signatures and documents are securely handled and stored in accordance with FDA safety regulations.
-
How does airSlate SignNow ensure compliance with FDA safety regulations?
airSlate SignNow implements multiple security measures to align with FDA safety requirements, including encryption and authentication protocols. This ensures that all documents are signed and stored securely, which is crucial for industries subject to strict regulatory oversight, such as healthcare and pharmaceuticals.
-
What features does airSlate SignNow offer to enhance FDA safety compliance?
Key features of airSlate SignNow that enhance FDA safety compliance include audit trails, secure document sharing, and customizable workflows. These tools help organizations track document history, maintain chain of custody, and ensure that all signature processes meet regulatory standards effectively.
-
Is airSlate SignNow cost-effective for businesses focused on FDA safety?
Yes, airSlate SignNow is designed to be a cost-effective solution for businesses looking to adhere to FDA safety standards. With competitive pricing plans, it provides the functionalities needed for compliant electronic signatures without a hefty investment, making it accessible for companies of all sizes.
-
Can I integrate airSlate SignNow with existing solutions for better FDA safety management?
Absolutely! airSlate SignNow offers seamless integrations with various software applications that are widely used in compliance and safety management, ensuring that your FDA safety protocols are well managed. This flexibility allows businesses to streamline their processes while still focusing on regulatory adherence.
-
How does airSlate SignNow help in tracking documents for FDA safety audits?
airSlate SignNow provides a comprehensive audit trail feature that is essential for tracking documents during FDA safety audits. This feature logs every action taken on a document, ensuring you have a complete history for compliance verification and audit readiness.
-
What are the benefits of using airSlate SignNow for industries regulated by FDA safety standards?
Using airSlate SignNow helps industries regulated by FDA safety standards streamline their document signing processes, reduce errors, and enhance compliance. It improves efficiency while ensuring that all electronic signatures and documents meet the critical requirements set forth by the FDA.
Get more for Fda 2541a
Find out other Fda 2541a
- Sign Tennessee Investment Contract Safe
- Sign Maryland Consulting Agreement Template Fast
- Sign California Distributor Agreement Template Myself
- How Do I Sign Louisiana Startup Business Plan Template
- Can I Sign Nevada Startup Business Plan Template
- Sign Rhode Island Startup Business Plan Template Now
- How Can I Sign Connecticut Business Letter Template
- Sign Georgia Business Letter Template Easy
- Sign Massachusetts Business Letter Template Fast
- Can I Sign Virginia Business Letter Template
- Can I Sign Ohio Startup Costs Budget Worksheet
- How Do I Sign Maryland 12 Month Sales Forecast
- How Do I Sign Maine Profit and Loss Statement
- How To Sign Wisconsin Operational Budget Template
- Sign North Carolina Profit and Loss Statement Computer
- Sign Florida Non-Compete Agreement Fast
- How Can I Sign Hawaii Non-Compete Agreement
- Sign Oklahoma General Partnership Agreement Online
- Sign Tennessee Non-Compete Agreement Computer
- Sign Tennessee Non-Compete Agreement Mobile