Food Drug Administration Animal 2021-2026


What is the Food Drug Administration Animal
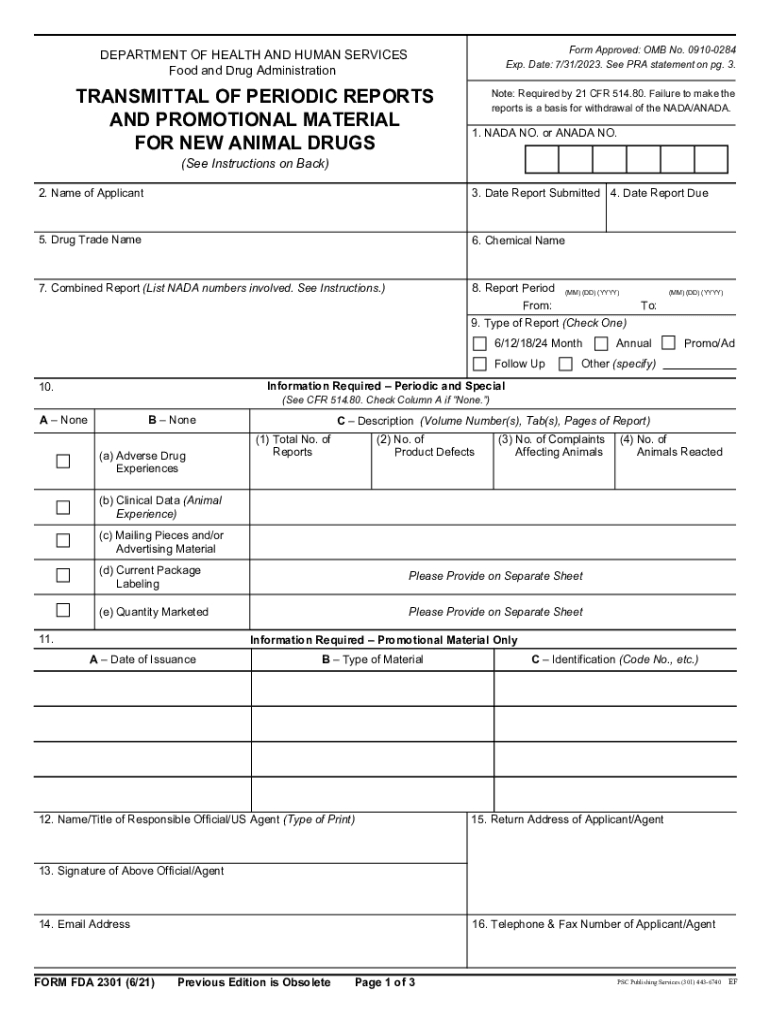
The Food Drug Administration Animal, often referred to in the context of regulatory compliance, pertains to the oversight of animal-related products and services by the FDA. This includes ensuring that animal drugs, feed, and veterinary devices are safe and effective for use. The FDA plays a crucial role in protecting public health by regulating the use of animals in research and the production of food products. Understanding this framework is essential for businesses and individuals involved in animal health and welfare.
Steps to complete the Food Drug Administration Animal
Completing the Food Drug Administration Animal form involves several key steps to ensure compliance and accuracy. Begin by gathering all necessary information, including details about the animal products or services involved. Next, carefully fill out the required sections of the form, paying close attention to the specific guidelines provided by the FDA. After completing the form, review it thoroughly to ensure all information is correct and complete. Finally, submit the form through the appropriate channels, whether online or via mail, as specified by the FDA.
Legal use of the Food Drug Administration Animal
The legal use of the Food Drug Administration Animal form is governed by various regulations that ensure compliance with federal laws. It is essential to understand the legal implications of using this form, especially in relation to animal health and safety standards. Compliance with these regulations not only protects public health but also safeguards businesses from potential legal issues. Utilizing a reliable eSignature solution can enhance the legal validity of the completed form, ensuring it meets all necessary requirements.
Required Documents
When completing the Food Drug Administration Animal form, certain documents may be required to support your submission. These typically include proof of compliance with relevant animal health regulations, documentation of product safety and efficacy, and any pertinent research data. Ensuring that all required documents are included with your submission can help facilitate a smoother review process by the FDA and reduce the likelihood of delays.
Form Submission Methods (Online / Mail / In-Person)
The Food Drug Administration Animal form can be submitted through various methods, depending on the specific requirements set forth by the FDA. Common submission methods include online submission via the FDA's designated portal, mailing the completed form to the appropriate FDA office, or delivering it in person. Each method has its own guidelines and processing times, so it is important to choose the one that best fits your needs and ensures timely compliance.
Who Issues the Form
The Food Drug Administration Animal form is issued by the FDA, which is responsible for regulating animal health products and services in the United States. This federal agency ensures that all submissions are evaluated based on established safety and efficacy standards. Understanding the role of the FDA in the issuance and regulation of this form is vital for compliance and successful navigation of the regulatory landscape.
Quick guide on how to complete food drug administration animal
Complete Food Drug Administration Animal effortlessly on any device
Web-based document management has become increasingly favored among businesses and individuals. It offers an ideal eco-friendly alternative to traditional printed and signed paperwork, allowing you to locate the necessary form and securely store it online. airSlate SignNow equips you with all the resources needed to create, edit, and eSign your documents swiftly without any holdups. Manage Food Drug Administration Animal on any device using airSlate SignNow Android or iOS applications and enhance any document-driven process today.
The easiest way to modify and eSign Food Drug Administration Animal with ease
- Obtain Food Drug Administration Animal and click on Get Form to begin.
- Employ the tools we offer to complete your document.
- Emphasize pertinent sections of the documents or obscure sensitive information using tools specifically provided by airSlate SignNow.
- Create your signature with the Sign tool, which takes mere seconds and holds the same legal validity as a conventional wet ink signature.
- Review all information and click on the Done button to preserve your updates.
- Select how you want to send your form: via email, SMS, invitation link, or download it to your computer.
Eliminate lost or misplaced paperwork, tedious form searching, or mistakes that necessitate printing new document copies. airSlate SignNow meets your document management needs in just a few clicks from any device of your choice. Alter and eSign Food Drug Administration Animal to ensure exceptional communication at every stage of the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Find and fill out the correct food drug administration animal
Create this form in 5 minutes!
People also ask
-
What is the 2301 form and why is it important for businesses?
The 2301 form is a key document used for various business processes, including electronic signatures and document management. Understanding how to effectively utilize the 2301 form can streamline your operations and ensure compliance with legal standards.
-
How does airSlate SignNow simplify the process of handling the 2301 form?
airSlate SignNow provides an easy-to-use platform that allows businesses to upload, send, and eSign the 2301 form efficiently. With its user-friendly interface, you can manage your documents without hassle, saving time and reducing paperwork.
-
What pricing plans are available for using airSlate SignNow for the 2301 form?
airSlate SignNow offers flexible pricing plans tailored to various business needs, ensuring affordability for handling the 2301 form. You can select a plan that fits your budget while gaining access to all necessary features for effective document management.
-
Can I integrate airSlate SignNow with other software while managing the 2301 form?
Yes, airSlate SignNow provides integrations with popular software applications, enhancing your ability to manage the 2301 form seamlessly. This allows for a more streamlined workflow by connecting your existing tools with our document signing platform.
-
What features does airSlate SignNow offer for optimizing the 2301 form process?
airSlate SignNow includes features such as templates, automated workflows, and tracking options that enhance the 2301 form handling process. These tools help ensure that your documents are completed accurately and efficiently.
-
How secure is airSlate SignNow when dealing with the 2301 form?
Security is a top priority for airSlate SignNow. When handling the 2301 form, your data is protected with advanced encryption protocols and complies with industry standards to keep your information safe.
-
Is it easy to eSign the 2301 form using airSlate SignNow?
Absolutely! airSlate SignNow makes it easier than ever to eSign the 2301 form with just a few clicks. Our platform allows users to sign documents digitally, eliminating the need for printing, scanning, and faxing.
Get more for Food Drug Administration Animal
- Agent of record letter sample 100271673 form
- Mold hold harmless form 238957706
- Exhibit cover sheet template 33051837 form
- Motion and order to set aside judgment forms and i
- Motion and order to set aside judgment forms and instructions courts oregon
- Maternal amp fetal care referral form barnes jewish hospital barnesjewish
- Sars patient contact log tool for logging health care staff caring for sars patients health ny form
- Cpap competency test scenarios form
Find out other Food Drug Administration Animal
- How To Sign Georgia Non-Profit Presentation
- Can I Sign Nevada Life Sciences PPT
- Help Me With Sign New Hampshire Non-Profit Presentation
- How To Sign Alaska Orthodontists Presentation
- Can I Sign South Dakota Non-Profit Word
- Can I Sign South Dakota Non-Profit Form
- How To Sign Delaware Orthodontists PPT
- How Can I Sign Massachusetts Plumbing Document
- How To Sign New Hampshire Plumbing PPT
- Can I Sign New Mexico Plumbing PDF
- How To Sign New Mexico Plumbing Document
- How To Sign New Mexico Plumbing Form
- Can I Sign New Mexico Plumbing Presentation
- How To Sign Wyoming Plumbing Form
- Help Me With Sign Idaho Real Estate PDF
- Help Me With Sign Idaho Real Estate PDF
- Can I Sign Idaho Real Estate PDF
- How To Sign Idaho Real Estate PDF
- How Do I Sign Hawaii Sports Presentation
- How Do I Sign Kentucky Sports Presentation