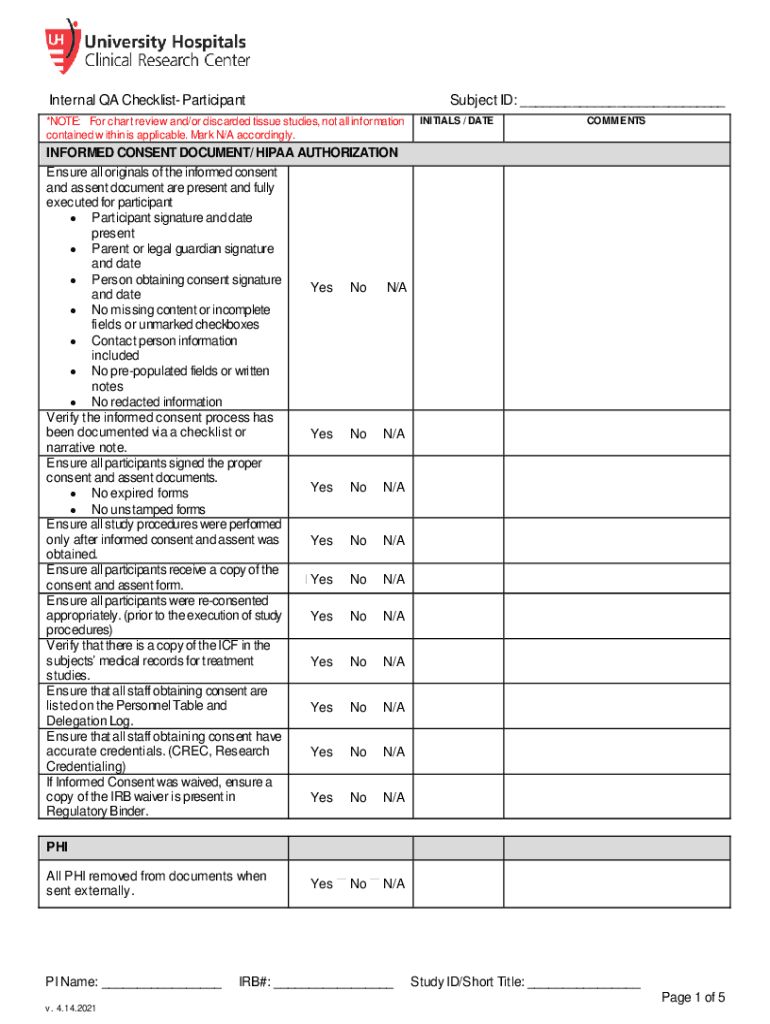
Internal QA Checklist Regulatory Study ID IRB# PI Name Form


What is the Internal QA Checklist Regulatory Study ID IRB# PI Name
The Internal QA Checklist Regulatory Study ID IRB# PI Name is a crucial document used in the context of clinical research and regulatory compliance. It serves as a systematic tool to ensure that all necessary quality assurance measures are in place for a study. This checklist includes various elements that help researchers and institutions verify that the study adheres to ethical standards and regulatory requirements. It typically encompasses sections related to the study protocol, informed consent processes, data management, and participant safety, among other critical areas.
How to use the Internal QA Checklist Regulatory Study ID IRB# PI Name
Using the Internal QA Checklist involves a step-by-step approach to ensure that all components of the research study meet regulatory standards. Begin by reviewing the checklist thoroughly to understand each section. As you progress through the study, systematically check off each item as it is completed. This practice not only helps in maintaining compliance but also facilitates communication among team members regarding the status of various tasks. Regular updates to the checklist can aid in identifying any areas that require additional attention or resources.
Key elements of the Internal QA Checklist Regulatory Study ID IRB# PI Name
Key elements of the Internal QA Checklist include:
- Study Protocol Compliance: Ensures that the study follows the approved protocol.
- Informed Consent: Verifies that participants are adequately informed about the study and their rights.
- Data Integrity: Confirms that data collection and management practices are robust and reliable.
- Participant Safety: Assesses measures in place to protect the health and well-being of study participants.
- Regulatory Documentation: Ensures that all necessary regulatory approvals and documents are in order.
Steps to complete the Internal QA Checklist Regulatory Study ID IRB# PI Name
Completing the Internal QA Checklist involves several important steps:
- Gather all relevant documents related to the study, including the study protocol and IRB approval.
- Review each section of the checklist carefully, ensuring that all items are addressed.
- Consult with team members to confirm that all aspects of the study are compliant.
- Document any discrepancies or areas needing improvement for future reference.
- Finalize the checklist by signing and dating it to indicate completion.
Legal use of the Internal QA Checklist Regulatory Study ID IRB# PI Name
The legal use of the Internal QA Checklist is essential for maintaining compliance with federal and state regulations governing clinical research. By adhering to the guidelines outlined in the checklist, researchers can demonstrate their commitment to ethical practices and participant safety. This documentation can serve as a legal safeguard in the event of audits or inquiries by regulatory bodies. It is advisable to retain copies of completed checklists as part of the study's official records.
Examples of using the Internal QA Checklist Regulatory Study ID IRB# PI Name
Examples of utilizing the Internal QA Checklist include:
- Conducting a pre-study meeting where the checklist is reviewed to ensure all team members understand their responsibilities.
- Using the checklist during site visits to verify compliance with regulatory standards.
- Incorporating feedback from the checklist into training sessions for new staff involved in the study.
Quick guide on how to complete internal qa checklist regulatory study id irb pi name
Effortlessly Prepare Internal QA Checklist Regulatory Study ID IRB# PI Name on Any Device
Managing documents online has gained traction among both businesses and individuals. It serves as an ideal eco-friendly alternative to traditional printed and signed papers, allowing you to easily locate the necessary form and securely store it online. airSlate SignNow equips you with all the resources needed to swiftly create, modify, and eSign your documents without any delays. Handle Internal QA Checklist Regulatory Study ID IRB# PI Name on any platform using airSlate SignNow's Android or iOS applications and simplify any document-related task today.
The Easiest Method to Modify and eSign Internal QA Checklist Regulatory Study ID IRB# PI Name Seamlessly
- Obtain Internal QA Checklist Regulatory Study ID IRB# PI Name and click Get Form to begin.
- Utilize the tools we offer to complete your document.
- Highlight pertinent sections of your documents or obscure sensitive information with the tools that airSlate SignNow has specifically designed for that purpose.
- Create your eSignature using the Sign feature, which takes mere seconds and carries the same legal validity as a traditional wet ink signature.
- Review all information carefully and then click the Done button to save your modifications.
- Select your preferred method for sending your form, whether it be by email, SMS, or invite link, or download it to your computer.
Eliminate concerns about missing or lost documents, tedious form searches, or mistakes that necessitate reprinting new copies. airSlate SignNow addresses your document management needs in just a few clicks from any device you choose. Adjust and eSign Internal QA Checklist Regulatory Study ID IRB# PI Name and guarantee outstanding communication at every stage of the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the internal qa checklist regulatory study id irb pi name
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is the Internal QA Checklist for Regulatory Study ID IRB# PI Name?
The Internal QA Checklist for Regulatory Study ID IRB# PI Name is a comprehensive tool designed to ensure compliance and quality assurance within your regulatory studies. It helps in tracking and verifying necessary documentation associated with the IRB process, focusing on accuracy and adherence to guidelines.
-
How can airSlate SignNow facilitate the Internal QA Checklist for Regulatory Study ID IRB# PI Name?
AirSlate SignNow offers a user-friendly platform that streamlines the management of the Internal QA Checklist for Regulatory Study ID IRB# PI Name. With our electronic signature capabilities, you can securely sign and share vital documents, ensuring timely and efficient completion of your compliance checks.
-
Are there any costs associated with using airSlate SignNow for the Internal QA Checklist for Regulatory Study ID IRB# PI Name?
Yes, airSlate SignNow provides various pricing plans to cater to different business needs. Each plan includes features that support the Internal QA Checklist for Regulatory Study ID IRB# PI Name, ensuring you get value for the investment while maintaining compliance standards.
-
What features support the Internal QA Checklist for Regulatory Study ID IRB# PI Name?
AirSlate SignNow includes features such as document templates, real-time tracking, and customizable workflows, all aimed at enhancing the management of the Internal QA Checklist for Regulatory Study ID IRB# PI Name. These features ensure that all necessary steps are accounted for and completed effectively.
-
How does airSlate SignNow integrate with other tools for the Internal QA Checklist for Regulatory Study ID IRB# PI Name?
AirSlate SignNow offers seamless integrations with multiple platforms such as Google Drive, Dropbox, and various CRM systems. This capability enhances the utility of the Internal QA Checklist for Regulatory Study ID IRB# PI Name, allowing for easier access and collaboration across different tools.
-
What benefits does airSlate SignNow provide for managing the Internal QA Checklist for Regulatory Study ID IRB# PI Name?
Using airSlate SignNow to manage the Internal QA Checklist for Regulatory Study ID IRB# PI Name brings signNow benefits, including enhanced efficiency, reduced time-to-complete processes, and improved compliance tracking. These advantages help your organization meet regulatory requirements without unnecessary delays.
-
Can I customize the Internal QA Checklist for Regulatory Study ID IRB# PI Name in airSlate SignNow?
Absolutely! AirSlate SignNow allows for full customization of the Internal QA Checklist for Regulatory Study ID IRB# PI Name, enabling you to tailor it according to your specific compliance needs. This flexibility ensures that your checklist is aligned with your study requirements and institutional guidelines.
Get more for Internal QA Checklist Regulatory Study ID IRB# PI Name
- Refrigeration contract for contractor arizona form
- Drainage contract for contractor arizona form
- Foundation contract for contractor arizona form
- Plumbing contract for contractor arizona form
- Brick mason contract for contractor arizona form
- Roofing contract for contractor arizona form
- Electrical contract for contractor arizona form
- Sheetrock drywall contract for contractor arizona form
Find out other Internal QA Checklist Regulatory Study ID IRB# PI Name
- Electronic signature Minnesota Legal LLC Operating Agreement Free
- Electronic signature Minnesota Legal LLC Operating Agreement Secure
- Electronic signature Louisiana Life Sciences LLC Operating Agreement Now
- Electronic signature Oregon Non-Profit POA Free
- Electronic signature South Dakota Non-Profit Business Plan Template Now
- Electronic signature South Dakota Non-Profit Lease Agreement Template Online
- Electronic signature Legal Document Missouri Online
- Electronic signature Missouri Legal Claim Online
- Can I Electronic signature Texas Non-Profit Permission Slip
- Electronic signature Missouri Legal Rental Lease Agreement Simple
- Electronic signature Utah Non-Profit Cease And Desist Letter Fast
- Electronic signature Missouri Legal Lease Agreement Template Free
- Electronic signature Non-Profit PDF Vermont Online
- Electronic signature Non-Profit PDF Vermont Computer
- Electronic signature Missouri Legal Medical History Mobile
- Help Me With Electronic signature West Virginia Non-Profit Business Plan Template
- Electronic signature Nebraska Legal Living Will Simple
- Electronic signature Nevada Legal Contract Safe
- How Can I Electronic signature Nevada Legal Operating Agreement
- How Do I Electronic signature New Hampshire Legal LLC Operating Agreement