INVESTIGATIONAL MEDICINAL PRODUCT DOSSIER 2016


What is the investigational medicinal product dossier?
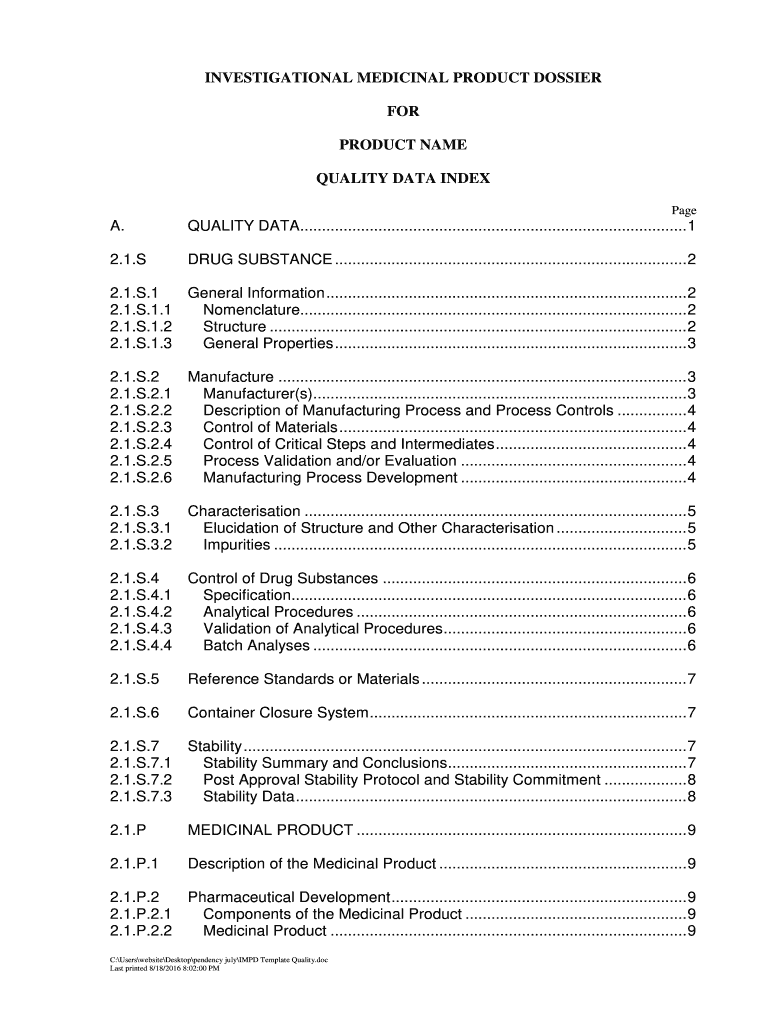
The investigational medicinal product dossier (IMPD) is a comprehensive document that provides essential information about an investigational medicinal product. It is a crucial component in the development of new drugs and is required for regulatory submissions. The dossier typically includes details such as the product's composition, manufacturing process, quality control measures, and preclinical and clinical data. It serves as a foundation for regulatory authorities to assess the safety and efficacy of the product before it can be tested in humans.
Key elements of the investigational medicinal product dossier
Several key elements are essential for a complete investigational medicinal product dossier. These include:
- Product description: Information about the active ingredients, formulation, and dosage form.
- Manufacturing details: Processes and controls used in the production of the medicinal product, including quality assurance measures.
- Preclinical data: Results from laboratory and animal studies that support the product's safety and efficacy.
- Clinical trial information: Protocols and data from clinical trials that demonstrate the product's effects in humans.
- Risk assessment: Evaluation of potential risks associated with the product and proposed mitigation strategies.
Steps to complete the investigational medicinal product dossier
Completing an investigational medicinal product dossier involves several important steps:
- Gather all necessary data on the product, including scientific literature and previous studies.
- Compile detailed information about the manufacturing process, including quality control measures.
- Document preclinical and clinical trial results, ensuring clarity and accuracy.
- Conduct a comprehensive risk assessment to identify and address potential safety concerns.
- Review and verify all information for compliance with regulatory requirements.
Legal use of the investigational medicinal product dossier
The investigational medicinal product dossier must comply with various legal and regulatory standards to be considered valid. In the United States, the Food and Drug Administration (FDA) oversees the submission and approval process for investigational products. Compliance with the FDA's guidelines ensures that the dossier meets legal requirements for safety, efficacy, and quality. Additionally, the use of electronic signatures and digital documentation must adhere to the ESIGN and UETA acts, which establish the legal validity of electronic records and signatures.
How to obtain the investigational medicinal product dossier
Obtaining an investigational medicinal product dossier typically involves collaboration with regulatory authorities and research institutions. Researchers or companies developing a new medicinal product must prepare the dossier based on their findings and data. They may also need to consult with regulatory experts to ensure that the dossier meets all necessary requirements. Once completed, the dossier can be submitted to the relevant regulatory body for review and approval before clinical trials can commence.
Quick guide on how to complete investigational medicinal product dossier
Effortlessly Prepare INVESTIGATIONAL MEDICINAL PRODUCT DOSSIER on Any Device
Managing documents online has gained signNow traction among businesses and individuals. It offers an excellent eco-friendly substitute for traditional printed and signed papers, allowing you to access the correct forms and securely store them online. airSlate SignNow equips you with all the tools necessary to create, edit, and electronically sign your documents swiftly and efficiently. Handle INVESTIGATIONAL MEDICINAL PRODUCT DOSSIER on any platform using airSlate SignNow's Android or iOS applications and enhance any document-based workflow today.
How to Modify and Electronically Sign INVESTIGATIONAL MEDICINAL PRODUCT DOSSIER with Ease
- Find INVESTIGATIONAL MEDICINAL PRODUCT DOSSIER and then click Get Form to begin.
- Utilize the tools we provide to complete your document.
- Mark pertinent sections of the documents or obscure sensitive information with tools specifically designed for that purpose by airSlate SignNow.
- Generate your electronic signature using the Sign tool, which takes mere seconds and carries the same legal validity as a conventional handwritten signature.
- Review all information and then click the Done button to preserve your changes.
- Choose your preferred method to send your form, whether by email, SMS, or invitation link, or download it to your computer.
Say goodbye to lost or misplaced files, tedious form searches, or errors that necessitate reprinting new document copies. airSlate SignNow takes care of all your document management needs in just a few clicks from any device you choose. Alter and electronically sign INVESTIGATIONAL MEDICINAL PRODUCT DOSSIER while ensuring exceptional communication at every stage of your form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Find and fill out the correct investigational medicinal product dossier
Create this form in 5 minutes!
How to create an eSignature for the investigational medicinal product dossier
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is an investigational medicinal product dossier?
An investigational medicinal product dossier (IMPD) provides detailed information about an experimental drug's quality, safety, and efficacy. It is essential for regulatory submissions and helps ensure compliance with the required standards during clinical trials.
-
How can airSlate SignNow assist with the management of an investigational medicinal product dossier?
airSlate SignNow streamlines the eSigning process for documents related to the investigational medicinal product dossier. The platform allows users to manage and secure all necessary documentation efficiently, ensuring that submissions are timely and organized.
-
What are the key features of airSlate SignNow for managing investigational medicinal product dossiers?
Key features of airSlate SignNow include user-friendly eSignature capabilities, document tracking, and secure storage. These features make it easier to collaborate on the investigational medicinal product dossier and maintain compliance with regulatory requirements.
-
Is airSlate SignNow suitable for both small and large organizations handling investigational medicinal product dossiers?
Yes, airSlate SignNow is designed to cater to businesses of all sizes, providing scalable solutions for managing investigational medicinal product dossiers. This flexibility allows organizations to adapt the platform to their specific needs, regardless of their scale.
-
What is the pricing structure for airSlate SignNow in relation to managing investigational medicinal product dossiers?
airSlate SignNow offers competitive pricing plans that provide excellent value for organizations managing investigational medicinal product dossiers. Each plan is tailored to different business needs, ensuring that you only pay for the features you require.
-
Can airSlate SignNow integrate with existing systems for handling investigational medicinal product dossiers?
Absolutely! airSlate SignNow can seamlessly integrate with various tools and platforms, enhancing the workflow for handling investigational medicinal product dossiers. These integrations help consolidate data management and streamline the documentation process.
-
What benefits does airSlate SignNow provide for clinical trial documentation, such as the investigational medicinal product dossier?
Using airSlate SignNow for clinical trial documentation, including the investigational medicinal product dossier, enhances efficiency, reduces turnaround times, and minimizes the risk of errors. This allows teams to focus on what matters most—successful trial execution.
Get more for INVESTIGATIONAL MEDICINAL PRODUCT DOSSIER
- Affidavit of amount due on open account form
- This day this cause came on for hearing on plaintiffs complaint for claim and form
- Claim and delivery south carolina judicial department form
- In the district court of payne county state of oklahoma form
- Partnership interests purchase agreement this partnership form
- Court of chancery rules delaware courts form
- 191820 in the supreme court of mississippi no 89 r form
- In the chancery court of tennessee for the thirtieth judicial form
Find out other INVESTIGATIONAL MEDICINAL PRODUCT DOSSIER
- eSign South Dakota Legal Letter Of Intent Free
- eSign Alaska Plumbing Memorandum Of Understanding Safe
- eSign Kansas Orthodontists Contract Online
- eSign Utah Legal Last Will And Testament Secure
- Help Me With eSign California Plumbing Business Associate Agreement
- eSign California Plumbing POA Mobile
- eSign Kentucky Orthodontists Living Will Mobile
- eSign Florida Plumbing Business Plan Template Now
- How To eSign Georgia Plumbing Cease And Desist Letter
- eSign Florida Plumbing Credit Memo Now
- eSign Hawaii Plumbing Contract Mobile
- eSign Florida Plumbing Credit Memo Fast
- eSign Hawaii Plumbing Claim Fast
- eSign Hawaii Plumbing Letter Of Intent Myself
- eSign Hawaii Plumbing Letter Of Intent Fast
- Help Me With eSign Idaho Plumbing Profit And Loss Statement
- eSign Illinois Plumbing Letter Of Intent Now
- eSign Massachusetts Orthodontists Last Will And Testament Now
- eSign Illinois Plumbing Permission Slip Free
- eSign Kansas Plumbing LLC Operating Agreement Secure