Navigating the Clinical Protocol Activation Process for Industry Form


Understanding the Clinical Protocol Activation Process
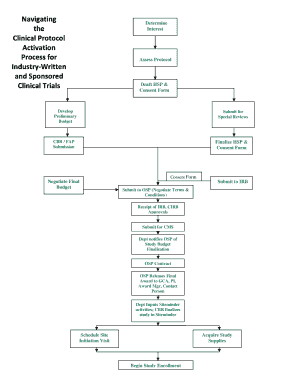
The clinical protocol activation process is a critical component in the healthcare and pharmaceutical industries, ensuring that clinical trials are conducted efficiently and ethically. This process involves several stages, including protocol development, regulatory review, and site activation. Each stage is designed to ensure compliance with federal regulations and to safeguard participant welfare. Understanding the intricacies of this process is essential for industry professionals aiming to navigate it successfully.
Steps to Complete the Clinical Protocol Activation Process
Completing the clinical protocol activation process involves a series of well-defined steps:
- Protocol Development: Draft a detailed clinical protocol that outlines the study's objectives, methodology, and participant criteria.
- Regulatory Submission: Submit the protocol to the relevant regulatory bodies, such as the Food and Drug Administration (FDA) or Institutional Review Boards (IRBs), for approval.
- Site Selection: Identify and select clinical sites where the trial will be conducted, ensuring they meet necessary regulatory and ethical standards.
- Site Activation: Complete all site-specific requirements, including training staff and obtaining necessary local approvals.
- Initiation Visit: Conduct an initiation visit at the site to ensure all parties understand the protocol and their responsibilities.
Required Documents for the Activation Process
Several key documents are essential for navigating the clinical protocol activation process effectively:
- Clinical Protocol: The main document detailing the study design and objectives.
- Informed Consent Forms: Documents that explain the study to participants and obtain their consent.
- Investigator's Brochure: A comprehensive document summarizing the clinical and non-clinical data on the investigational product.
- Regulatory Submission Documents: Include all forms and correspondence submitted to regulatory bodies.
- Site Agreements: Contracts that outline the terms between the sponsor and clinical site.
Legal Considerations in the Clinical Protocol Activation Process
Legal compliance is paramount in the clinical protocol activation process. This includes adherence to federal regulations, such as those enforced by the FDA, as well as state-specific laws governing clinical trials. Ensuring that all documentation is accurate and complete helps mitigate the risk of legal issues. Additionally, maintaining participant confidentiality and obtaining informed consent are legal obligations that must be strictly followed throughout the process.
Examples of Clinical Protocol Activation
Understanding practical examples can provide clarity on the clinical protocol activation process. For instance, a pharmaceutical company may initiate a trial for a new medication. They would begin by drafting a clinical protocol, followed by submitting it to the FDA for approval. Once approved, they would select clinical sites, train the staff, and conduct the necessary initiation visits. Each step is documented to ensure compliance and facilitate smooth operations throughout the trial.
Digital Tools for Managing the Activation Process
Utilizing digital tools can significantly enhance the efficiency of the clinical protocol activation process. Electronic signature solutions, like those offered by signNow, streamline the signing of essential documents, reducing the time spent on paperwork. These tools also improve collaboration among team members and ensure that all documents are securely stored and easily accessible. This digital approach not only saves time but also enhances compliance with regulatory requirements.
Quick guide on how to complete navigating the clinical protocol activation process for industry
Complete [SKS] effortlessly on any device
Online document management has surged in popularity among businesses and individuals. It serves as an ideal eco-friendly alternative to conventional printed and signed documents, allowing you to access the necessary forms and securely keep them online. airSlate SignNow equips you with all the tools needed to create, modify, and electronically sign your documents quickly and efficiently. Handle [SKS] on any device using the airSlate SignNow Android or iOS apps and enhance any document-based process today.
The easiest way to modify and electronically sign [SKS] smoothly
- Find [SKS] and click Get Form to commence.
- Utilize the tools we provide to fill out your form.
- Highlight important sections of your documents or redact sensitive information using the tools that airSlate SignNow offers specifically for this purpose.
- Create your electronic signature with the Sign tool, which takes mere seconds and carries the same legal validity as a traditional handwritten signature.
- Review the details and click the Done button to save your changes.
- Choose how you wish to send your form: via email, SMS, or an invitation link, or download it to your computer.
Eliminate concerns of lost or misplaced documents, tedious form searches, or mistakes necessitating the printing of new document copies. airSlate SignNow fulfills all your document management needs in just a few clicks from any device you prefer. Modify and electronically sign [SKS] and ensure excellent communication throughout your form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Related searches to Navigating The Clinical Protocol Activation Process For Industry
Create this form in 5 minutes!
How to create an eSignature for the navigating the clinical protocol activation process for industry
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is involved in Navigating The Clinical Protocol Activation Process For Industry?
Navigating The Clinical Protocol Activation Process For Industry involves a series of steps to ensure that clinical protocols are set up effectively. This process includes obtaining necessary approvals, integrating with existing systems, and ensuring compliance with regulations. With airSlate SignNow, these steps become streamlined, enabling quicker, efficient transitions.
-
How can airSlate SignNow assist in Navigating The Clinical Protocol Activation Process For Industry?
airSlate SignNow provides a comprehensive platform that simplifies Navigating The Clinical Protocol Activation Process For Industry. Our user-friendly interface allows users to easily send, eSign, and manage documents, ensuring that all necessary approvals are obtained efficiently. This not only speeds up the process but also enhances compliance and traceability.
-
What features does airSlate SignNow offer for Navigating The Clinical Protocol Activation Process For Industry?
airSlate SignNow offers features such as customizable templates, automated workflows, and secure eSignature capabilities. These tools are designed to facilitate Navigating The Clinical Protocol Activation Process For Industry by making document management simpler and more efficient. Additionally, the platform integrates seamlessly with various software to enhance your workflow.
-
What are the pricing options for airSlate SignNow when Navigating The Clinical Protocol Activation Process For Industry?
airSlate SignNow offers flexible pricing plans that cater to different business needs while Navigating The Clinical Protocol Activation Process For Industry. You can choose from monthly or annual subscriptions, ensuring that you only pay for the features you need. Our plans are designed to be cost-effective, allowing you to scale as your requirements grow.
-
Is airSlate SignNow compliant with industry regulations for Navigating The Clinical Protocol Activation Process For Industry?
Yes, airSlate SignNow is designed to be compliant with major industry regulations essential for Navigating The Clinical Protocol Activation Process For Industry. Our platform follows strict security protocols to ensure data integrity and confidentiality. Users can be confident that their document management practices adhere to necessary compliance standards.
-
How does airSlate SignNow integrate with other tools while Navigating The Clinical Protocol Activation Process For Industry?
airSlate SignNow offers robust integration capabilities with popular applications like Google Workspace, Salesforce, and Microsoft Office. This means that while Navigating The Clinical Protocol Activation Process For Industry, you can easily connect your existing systems with airSlate SignNow to ensure a seamless flow of information. Our API also allows for custom integration solutions.
-
What benefits does airSlate SignNow provide when Navigating The Clinical Protocol Activation Process For Industry?
The main benefits of using airSlate SignNow when Navigating The Clinical Protocol Activation Process For Industry include increased efficiency, enhanced compliance, and reduced turnaround times. Our platform streamlines workflows and allows for quick document handling, so you can focus on the critical aspects of your clinical protocols without delays. This leads to faster project timelines and improved communication.
Get more for Navigating The Clinical Protocol Activation Process For Industry
Find out other Navigating The Clinical Protocol Activation Process For Industry
- How To Sign Ohio Government Form
- Help Me With Sign Washington Government Presentation
- How To Sign Maine Healthcare / Medical PPT
- How Do I Sign Nebraska Healthcare / Medical Word
- How Do I Sign Washington Healthcare / Medical Word
- How Can I Sign Indiana High Tech PDF
- How To Sign Oregon High Tech Document
- How Do I Sign California Insurance PDF
- Help Me With Sign Wyoming High Tech Presentation
- How Do I Sign Florida Insurance PPT
- How To Sign Indiana Insurance Document
- Can I Sign Illinois Lawers Form
- How To Sign Indiana Lawers Document
- How To Sign Michigan Lawers Document
- How To Sign New Jersey Lawers PPT
- How Do I Sign Arkansas Legal Document
- How Can I Sign Connecticut Legal Document
- How Can I Sign Indiana Legal Form
- Can I Sign Iowa Legal Document
- How Can I Sign Nebraska Legal Document