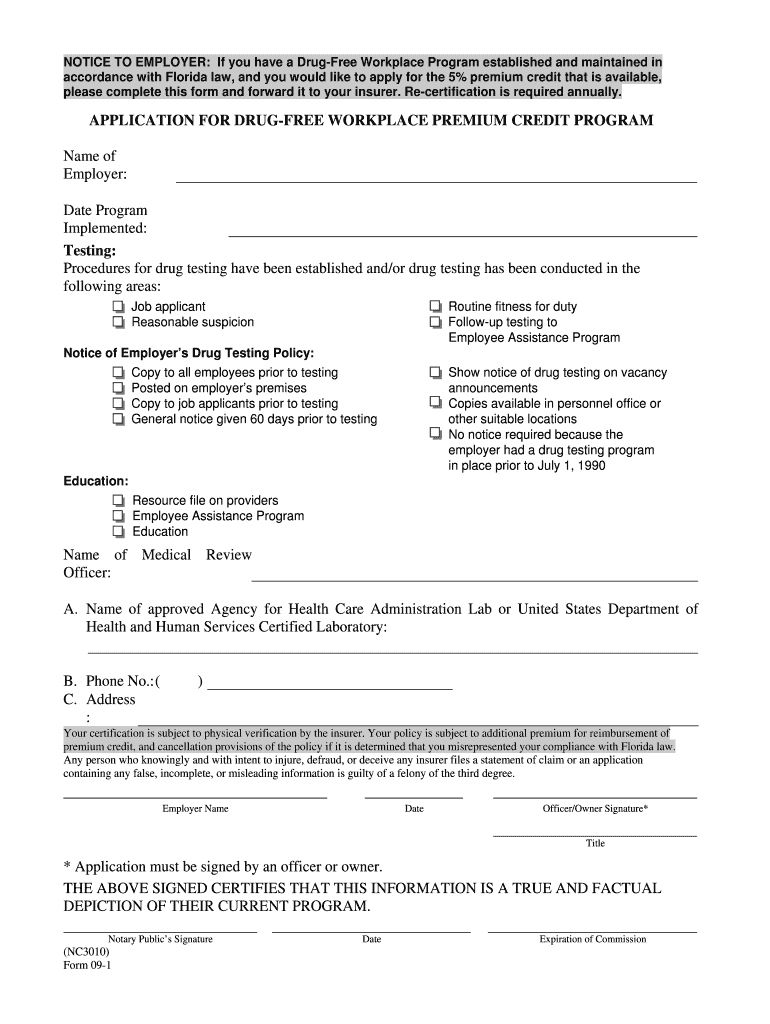
Drug Application Form09 1A Effective 110119 1


What is the Drug Application Form09 1A Effective 110119 1
The Drug Application Form09 1A Effective 110119 1 is a specialized document used in the pharmaceutical industry to apply for the approval of new drugs or modifications to existing drugs. This form is essential for regulatory compliance and is typically submitted to the relevant authorities overseeing drug safety and efficacy. It includes detailed information about the drug's composition, intended use, and clinical trial results, ensuring that all necessary data is presented for evaluation.
How to use the Drug Application Form09 1A Effective 110119 1
Using the Drug Application Form09 1A Effective 110119 1 involves several key steps. First, gather all required documentation, including research data and safety information. Next, complete the form with accurate and thorough details about the drug. After filling out the form, review it carefully to ensure all information is correct and complete. Finally, submit the form to the appropriate regulatory agency, either electronically or via mail, depending on the submission guidelines provided by that agency.
Steps to complete the Drug Application Form09 1A Effective 110119 1
Completing the Drug Application Form09 1A Effective 110119 1 requires a systematic approach:
- Gather all necessary documents, including clinical trial data and safety reports.
- Fill out the form with precise and comprehensive information about the drug.
- Ensure that all sections of the form are completed, including any required signatures.
- Review the form for accuracy and completeness before submission.
- Submit the form according to the specified guidelines, ensuring compliance with all regulations.
Legal use of the Drug Application Form09 1A Effective 110119 1
The legal use of the Drug Application Form09 1A Effective 110119 1 is governed by various regulations that ensure the safety and efficacy of pharmaceuticals. To be legally binding, the form must be completed accurately and submitted to the appropriate regulatory body. Compliance with the relevant laws and guidelines is critical, as failure to adhere to these requirements can result in penalties or rejection of the application. Understanding these legal frameworks is essential for successful drug approval.
Key elements of the Drug Application Form09 1A Effective 110119 1
Key elements of the Drug Application Form09 1A Effective 110119 1 include:
- Drug identification details, including name and formulation.
- Information on the manufacturer and production processes.
- Clinical trial results demonstrating the drug's safety and efficacy.
- Labeling information that complies with regulatory standards.
- Proposed uses and indications for the drug.
Eligibility Criteria
Eligibility to submit the Drug Application Form09 1A Effective 110119 1 typically includes being a registered pharmaceutical company or research entity with the capability to conduct clinical trials. The applicant must also have completed all necessary preclinical and clinical studies to provide sufficient evidence of the drug's safety and effectiveness. Additionally, the applicant must comply with all regulatory requirements set forth by the relevant authorities.
Quick guide on how to complete drug free application form09 1a effective 110119 1
Effortlessly Complete Drug Application Form09 1A Effective 110119 1 on Any Device
Digital document management has gained signNow traction among businesses and individuals. It offers an ideal environmentally friendly substitute to traditional printed and signed documents, allowing you to obtain the correct format and securely store it online. airSlate SignNow equips you with all the necessary tools to create, edit, and eSign your documents swiftly without any hold-ups. Handle Drug Application Form09 1A Effective 110119 1 on any device using the airSlate SignNow apps for Android or iOS and simplify any document-related process today.
How to Edit and eSign Drug Application Form09 1A Effective 110119 1 with Ease
- Find Drug Application Form09 1A Effective 110119 1 and click Acquire Form to begin.
- Utilize the tools we provide to complete your document.
- Emphasize pertinent parts of your documents or redact sensitive information with tools that airSlate SignNow offers specifically for that purpose.
- Generate your signature with the Sign feature, which takes mere seconds and holds the same legal validity as a conventional wet ink signature.
- Review all details and click on the Finish button to save your changes.
- Select how you would like to share your form, via email, text message (SMS), or invitation link, or download it to your computer.
Eliminate concerns about lost or misplaced documents, time-consuming form searches, or mistakes that require new copies to be printed. airSlate SignNow addresses all your document management needs in just a few clicks from any device you prefer. Edit and eSign Drug Application Form09 1A Effective 110119 1 and ensure excellent communication at any stage of your document preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
People also ask
-
What is the Drug Application Form09 1A Effective 110119 1?
The Drug Application Form09 1A Effective 110119 1 is a standardized document used in the pharmaceutical industry for submitting drug applications. This form is critical for ensuring compliance with regulatory standards, allowing for efficient processing of drug approvals.
-
How can airSlate SignNow enhance the use of the Drug Application Form09 1A Effective 110119 1?
airSlate SignNow allows users to easily eSign the Drug Application Form09 1A Effective 110119 1 securely and efficiently. Our platform streamlines the signing process, ensuring that your application is submitted promptly and with minimal hassle.
-
Is there a cost associated with using airSlate SignNow for the Drug Application Form09 1A Effective 110119 1?
Yes, airSlate SignNow offers competitive pricing plans that cater to various business needs. You can choose from different subscription tiers to find the right solution for handling the Drug Application Form09 1A Effective 110119 1, ensuring you pay only for what you need.
-
What features does airSlate SignNow offer for managing the Drug Application Form09 1A Effective 110119 1?
Our platform includes features such as customizable templates, automated workflows, and secure document storage. These tools are designed to simplify the management of the Drug Application Form09 1A Effective 110119 1, making the entire process more efficient.
-
Can airSlate SignNow integrate with other software for the Drug Application Form09 1A Effective 110119 1?
Absolutely! airSlate SignNow seamlessly integrates with a variety of popular software applications. This allows you to manage the Drug Application Form09 1A Effective 110119 1 alongside your existing systems for enhanced productivity.
-
What are the benefits of using airSlate SignNow for the Drug Application Form09 1A Effective 110119 1?
Using airSlate SignNow for the Drug Application Form09 1A Effective 110119 1 offers numerous benefits, including faster processing times, reduced errors, and increased compliance. Our platform simplifies communication with relevant stakeholders, ensuring a smoother application process.
-
Is airSlate SignNow secure for handling the Drug Application Form09 1A Effective 110119 1?
Yes, airSlate SignNow prioritizes security and compliance. With advanced encryption and authentication protocols, you can trust that the Drug Application Form09 1A Effective 110119 1 and other confidential documents are protected from unauthorized access.
Get more for Drug Application Form09 1A Effective 110119 1
- Text messaging and email messaging consent form
- Ratio worksheet printable math worksheets for kids form
- Export declaration form
- Dincel order form
- Form 15 h oriental bank of commerce
- Prom outside guest form nation ford high nation ford high school nfhs fort mill k12 sc
- Gst524 gsthst new residential rental property rebate form
- Diy livery contract template form
Find out other Drug Application Form09 1A Effective 110119 1
- How Can I Electronic signature Wyoming Real Estate Quitclaim Deed
- Electronic signature Virginia Police Quitclaim Deed Secure
- How Can I Electronic signature West Virginia Police Letter Of Intent
- How Do I Electronic signature Washington Police Promissory Note Template
- Electronic signature Wisconsin Police Permission Slip Free
- Electronic signature Minnesota Sports Limited Power Of Attorney Fast
- Electronic signature Alabama Courts Quitclaim Deed Safe
- How To Electronic signature Alabama Courts Stock Certificate
- Can I Electronic signature Arkansas Courts Operating Agreement
- How Do I Electronic signature Georgia Courts Agreement
- Electronic signature Georgia Courts Rental Application Fast
- How Can I Electronic signature Hawaii Courts Purchase Order Template
- How To Electronic signature Indiana Courts Cease And Desist Letter
- How Can I Electronic signature New Jersey Sports Purchase Order Template
- How Can I Electronic signature Louisiana Courts LLC Operating Agreement
- How To Electronic signature Massachusetts Courts Stock Certificate
- Electronic signature Mississippi Courts Promissory Note Template Online
- Electronic signature Montana Courts Promissory Note Template Now
- Electronic signature Montana Courts Limited Power Of Attorney Safe
- Electronic signature Oklahoma Sports Contract Safe