ICH GCP Essential Document Checklist Welcome to URMC Urmc Rochester Form


What is the ICH GCP Essential Document Checklist?
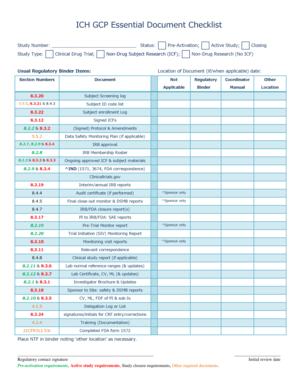
The ICH GCP Essential Document Checklist is a comprehensive guide designed to ensure that all necessary documents are prepared and maintained throughout the clinical trial process. This checklist is crucial for compliance with the International Council for Harmonisation (ICH) Good Clinical Practice (GCP) guidelines, which aim to protect the rights, safety, and well-being of trial participants while ensuring the integrity of clinical trial data. The checklist typically includes documents such as trial protocols, informed consent forms, and investigator brochures, among others, which are essential for regulatory submissions and audits.
Key elements of the ICH GCP Essential Document Checklist
Understanding the key elements of the ICH GCP Essential Document Checklist is vital for researchers and sponsors. The checklist often includes:
- Trial Protocol: A detailed plan outlining the objectives, design, methodology, and statistical considerations of the trial.
- Informed Consent Forms: Documents that ensure participants are fully informed about the trial and their rights.
- Investigator Brochure: A comprehensive document that provides clinical and non-clinical data on the investigational product.
- Case Report Forms: Tools used to collect data from each participant during the trial.
- Regulatory Approvals: Documentation showing that the trial has received necessary approvals from regulatory bodies.
Steps to complete the ICH GCP Essential Document Checklist
Completing the ICH GCP Essential Document Checklist involves several important steps:
- Gather Required Documents: Collect all necessary documents as outlined in the checklist.
- Review for Completeness: Ensure that all documents are complete, signed, and dated as required.
- Organize Documents: Arrange the documents in the order specified by the checklist for easy access and review.
- Maintain Version Control: Keep track of document versions to ensure that the most current information is used.
- Conduct Regular Audits: Schedule periodic reviews to ensure compliance with the checklist and regulatory requirements.
Legal use of the ICH GCP Essential Document Checklist
The legal use of the ICH GCP Essential Document Checklist is fundamental for maintaining compliance with regulatory standards. Ensuring that all documents are properly executed and stored can protect against legal challenges and audits. The checklist serves as a record of compliance with GCP guidelines, which can be crucial in demonstrating adherence to ethical standards in clinical research. Additionally, proper documentation can safeguard against potential penalties associated with non-compliance.
How to use the ICH GCP Essential Document Checklist
Using the ICH GCP Essential Document Checklist effectively involves integrating it into the clinical trial workflow. Researchers should familiarize themselves with the checklist at the trial's outset, ensuring that all team members understand their responsibilities regarding documentation. Regular training sessions can help reinforce the importance of the checklist and ensure that everyone is aware of any updates or changes. Utilizing digital tools can streamline the process, making it easier to track document completion and compliance.
Examples of using the ICH GCP Essential Document Checklist
Practical examples of using the ICH GCP Essential Document Checklist can illustrate its importance in real-world scenarios. For instance, during a clinical trial for a new medication, the checklist may help ensure that all informed consent forms are collected and stored securely, protecting participant rights. Additionally, if an audit occurs, having a well-maintained checklist can facilitate a smoother review process, demonstrating that the trial adhered to all necessary protocols and regulations.
Quick guide on how to complete ich gcp essential document checklist welcome to urmc urmc rochester
Effortlessly Prepare ICH GCP Essential Document Checklist Welcome To URMC Urmc Rochester on Any Device
Digital document management has gained traction among businesses and individuals. It offers an ideal environmentally-friendly substitute for conventional printed and signed documentation, allowing you to access the correct form and securely save it online. airSlate SignNow equips you with all the necessary tools to create, edit, and eSign your documents swiftly without delays. Manage ICH GCP Essential Document Checklist Welcome To URMC Urmc Rochester on any platform using airSlate SignNow's Android or iOS applications and streamline your document-related tasks today.
How to Edit and eSign ICH GCP Essential Document Checklist Welcome To URMC Urmc Rochester with Ease
- Obtain ICH GCP Essential Document Checklist Welcome To URMC Urmc Rochester and click on Get Form to begin.
- Utilize the tools we offer to complete your document.
- Emphasize important sections of the documents or conceal sensitive information with tools specifically designed for that purpose by airSlate SignNow.
- Generate your eSignature using the Sign feature, which takes seconds and holds the same legal validity as a traditional wet ink signature.
- Review all the details and click on the Done button to retain your modifications.
- Choose how you wish to send your form, via email, SMS, or a shared link, or download it to your computer.
Eliminate concerns about lost or misplaced documents, cumbersome form searching, or mistakes that require printing new document copies. airSlate SignNow fulfills all your document management needs in just a few clicks from any device you prefer. Edit and eSign ICH GCP Essential Document Checklist Welcome To URMC Urmc Rochester to ensure effective communication throughout the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Create this form in 5 minutes!
How to create an eSignature for the ich gcp essential document checklist welcome to urmc urmc rochester
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is included in an essential documents checklist?
An essential documents checklist typically includes items such as contracts, NDAs, and compliance forms that are critical for business operations. By using airSlate SignNow, you can ensure that all necessary documents are easily accessible and securely managed. This checklist can help streamline document preparation and enhance your efficiency when managing paperwork.
-
How does airSlate SignNow help with my essential documents checklist?
airSlate SignNow simplifies the process of creating and managing your essential documents checklist by allowing you to create templates for frequently used forms. This way, you can save time and reduce errors in document preparation. With our platform, you can easily track, send, and eSign documents in one place.
-
What are the pricing options for airSlate SignNow?
Pricing for airSlate SignNow is designed to be cost-effective, featuring various plans to accommodate different business needs. Each plan provides access to features that enhance your essential documents checklist management. Check our pricing page for the latest updates and find a plan that aligns with your requirements.
-
Can I integrate airSlate SignNow with other applications to manage my essential documents checklist?
Yes, airSlate SignNow offers robust integrations with numerous applications like Google Drive, Salesforce, and many others. These integrations can help streamline your workflow when dealing with your essential documents checklist. This connectivity ensures seamless operations across different platforms, making document management more efficient.
-
What benefits does using an essential documents checklist provide?
An essential documents checklist helps ensure that all critical documents are accounted for, reducing the chance of missed or lost paperwork. It also enhances workflow by making it easier to track document statuses within airSlate SignNow. Ultimately, this leads to improved productivity and a more organized approach to document management.
-
Is airSlate SignNow user-friendly for managing an essential documents checklist?
Absolutely! airSlate SignNow is designed with user experience in mind, making it easy for anyone to manage their essential documents checklist with minimal training. Our intuitive interface allows users to quickly navigate the features and automate tasks, ensuring a smooth document management experience for all team members.
-
How does airSlate SignNow ensure the security of my essential documents checklist?
Security is a top priority at airSlate SignNow. We implement advanced encryption protocols and secure access controls to protect your essential documents checklist and any sensitive information contained within. Rest assured that your documents are safeguarded while you manage them efficiently through our platform.
Get more for ICH GCP Essential Document Checklist Welcome To URMC Urmc Rochester
- Pit cg form
- Pdf affidavit of gift of motor vehicle or boat form
- About form 1040 ss us self employment tax return
- 2020 form 1040 pr federal self employment contribution statement for residents of puerto rico
- 2020 instructions for form 1120 s internal revenue service
- Content disposition http mdn 549395077 form
- 2020 instructions for form 1065 instructions for form 1065 us return of partnership income
- Get the free new mexico tax form pit 1 2019 2021 pdffiller
Find out other ICH GCP Essential Document Checklist Welcome To URMC Urmc Rochester
- Can I eSign Nebraska Police Form
- Can I eSign Nebraska Courts PDF
- How Can I eSign North Carolina Courts Presentation
- How Can I eSign Washington Police Form
- Help Me With eSignature Tennessee Banking PDF
- How Can I eSignature Virginia Banking PPT
- How Can I eSignature Virginia Banking PPT
- Can I eSignature Washington Banking Word
- Can I eSignature Mississippi Business Operations Document
- How To eSignature Missouri Car Dealer Document
- How Can I eSignature Missouri Business Operations PPT
- How Can I eSignature Montana Car Dealer Document
- Help Me With eSignature Kentucky Charity Form
- How Do I eSignature Michigan Charity Presentation
- How Do I eSignature Pennsylvania Car Dealer Document
- How To eSignature Pennsylvania Charity Presentation
- Can I eSignature Utah Charity Document
- How Do I eSignature Utah Car Dealer Presentation
- Help Me With eSignature Wyoming Charity Presentation
- How To eSignature Wyoming Car Dealer PPT