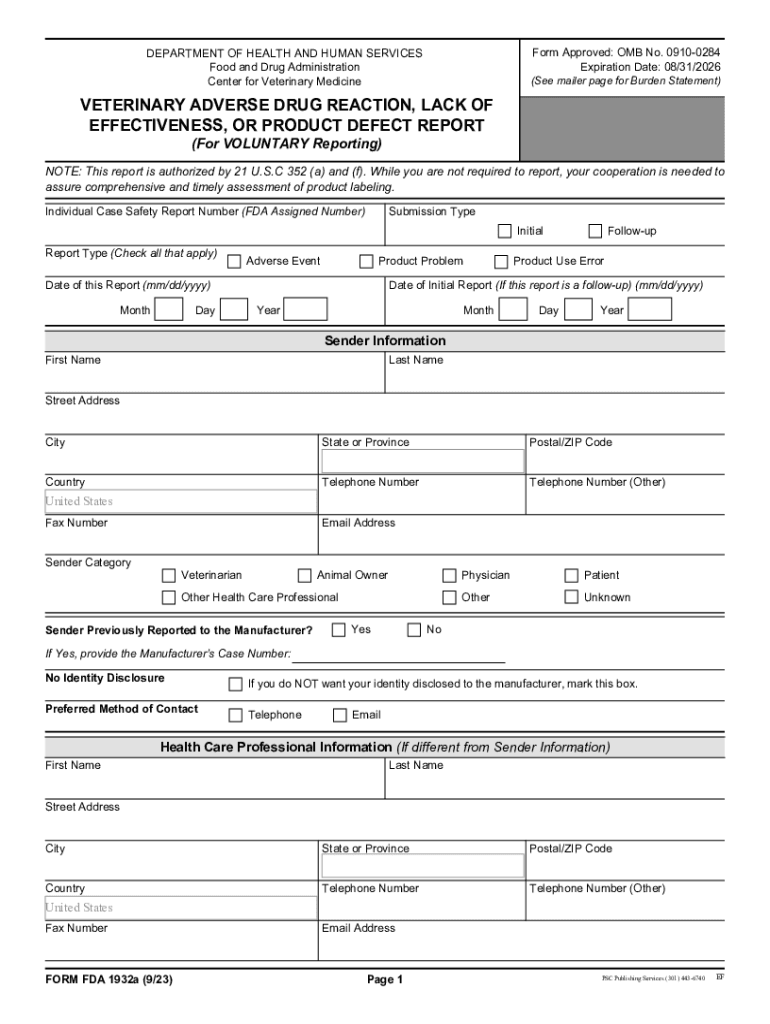
FORM FDA 1932a Veterinary Adverse Drug Reaction, Lack of Effectiveness, or Product Defect Report 2023-2026


What is the FORM FDA 1932a Veterinary Adverse Drug Reaction, Lack Of Effectiveness, Or Product Defect Report
The FORM FDA 1932a is a critical document used in the veterinary field to report adverse drug reactions, lack of effectiveness, or product defects associated with veterinary drugs. This form is essential for ensuring the safety and efficacy of veterinary medications. It allows veterinarians, animal owners, and manufacturers to communicate potential issues that could affect animal health or product performance. By collecting this information, the FDA can monitor drug safety and take necessary actions to protect animal welfare and public health.
How to use the FORM FDA 1932a Veterinary Adverse Drug Reaction, Lack Of Effectiveness, Or Product Defect Report
Using the FORM FDA 1932a involves several straightforward steps. First, gather all relevant information regarding the adverse event or product defect, including details about the animal, the medication administered, and the nature of the reaction or defect. Next, complete the form accurately, ensuring all sections are filled out to provide a comprehensive report. Once completed, the form can be submitted to the appropriate FDA office, either electronically or by mail, depending on the submission guidelines provided by the FDA.
Steps to complete the FORM FDA 1932a Veterinary Adverse Drug Reaction, Lack Of Effectiveness, Or Product Defect Report
Completing the FORM FDA 1932a requires careful attention to detail. Follow these steps:
- Gather Information: Collect all necessary data regarding the adverse event, including the animal's species, age, and health status.
- Fill Out the Form: Enter information in each section, including the drug name, dosage, and any observed reactions.
- Provide Context: Describe the circumstances surrounding the event, such as the treatment regimen and any other medications involved.
- Review the Form: Ensure all information is accurate and complete before submission.
- Submit the Form: Send the completed form to the FDA as per the specified submission method.
Key elements of the FORM FDA 1932a Veterinary Adverse Drug Reaction, Lack Of Effectiveness, Or Product Defect Report
The FORM FDA 1932a includes several key elements that are crucial for effective reporting. These elements typically encompass:
- Identifying Information: Details about the reporting individual, including name, contact information, and role (e.g., veterinarian, pet owner).
- Animal Information: Data regarding the animal affected, such as species, breed, age, and health history.
- Drug Information: Specifics about the drug involved, including name, dosage, and administration method.
- Adverse Reaction Details: A description of the reaction or defect observed, including severity and duration.
- Additional Comments: Any other relevant information that may assist in understanding the context of the report.
Legal use of the FORM FDA 1932a Veterinary Adverse Drug Reaction, Lack Of Effectiveness, Or Product Defect Report
The legal use of the FORM FDA 1932a is vital for compliance with federal regulations governing veterinary drug safety. Submitting this form is not only a best practice but also a legal obligation for veterinarians and manufacturers when they encounter adverse reactions or product defects. Proper reporting helps ensure that the FDA can take necessary regulatory actions, which may include product recalls or safety updates. Failure to report can lead to legal repercussions and compromise animal health and safety.
How to obtain the FORM FDA 1932a Veterinary Adverse Drug Reaction, Lack Of Effectiveness, Or Product Defect Report
The FORM FDA 1932a can be obtained directly from the FDA's official website or through veterinary regulatory resources. It is available in various formats, including PDF, which allows for easy downloading and printing. Additionally, veterinarians and animal health professionals may access the form through professional organizations or regulatory agencies that provide guidance on veterinary drug safety reporting.
Quick guide on how to complete form fda 1932a veterinary adverse drug reaction lack of effectiveness or product defect report
Effortlessly Prepare FORM FDA 1932a Veterinary Adverse Drug Reaction, Lack Of Effectiveness, Or Product Defect Report on Any Device
The management of online documents has become increasingly popular among companies and individuals alike. It offers an ideal environmentally friendly alternative to traditional printed and signed documents since you can locate the appropriate form and securely store it online. airSlate SignNow equips you with all the necessary tools to create, modify, and electronically sign your documents swiftly without complications. Handle FORM FDA 1932a Veterinary Adverse Drug Reaction, Lack Of Effectiveness, Or Product Defect Report on any device using airSlate SignNow's Android or iOS applications and enhance any document-related process today.
Steps to Modify and Electronically Sign FORM FDA 1932a Veterinary Adverse Drug Reaction, Lack Of Effectiveness, Or Product Defect Report Seamlessly
- Obtain FORM FDA 1932a Veterinary Adverse Drug Reaction, Lack Of Effectiveness, Or Product Defect Report and click Get Form to begin.
- Utilize the tools available to complete your document.
- Emphasize important sections of the documents or conceal sensitive information with the tools that airSlate SignNow specifically provides for that purpose.
- Create your electronic signature using the Sign tool, which takes only seconds and carries the same legal validity as a conventional wet ink signature.
- Review all the information and click on the Done button to save your changes.
- Select your preferred method of delivery for your form, whether by email, SMS, invitation link, or download it to your computer.
Say goodbye to lost or misfiled documents, the hassle of searching for forms, or errors that necessitate printing new copies. airSlate SignNow meets your document management needs in just a few clicks from any device you prefer. Modify and electronically sign FORM FDA 1932a Veterinary Adverse Drug Reaction, Lack Of Effectiveness, Or Product Defect Report and ensure effective communication at every stage of the form preparation process with airSlate SignNow.
Create this form in 5 minutes or less
Find and fill out the correct form fda 1932a veterinary adverse drug reaction lack of effectiveness or product defect report
Create this form in 5 minutes!
How to create an eSignature for the form fda 1932a veterinary adverse drug reaction lack of effectiveness or product defect report
How to create an electronic signature for a PDF online
How to create an electronic signature for a PDF in Google Chrome
How to create an e-signature for signing PDFs in Gmail
How to create an e-signature right from your smartphone
How to create an e-signature for a PDF on iOS
How to create an e-signature for a PDF on Android
People also ask
-
What is veterinary fda1932a signnow?
Veterinary fda1932a signnow is a specialized electronic signature solution designed for veterinary practices to streamline their document management. It allows veterinarians to send and eSign important documents quickly and securely, ensuring compliance with industry regulations.
-
How does veterinary fda1932a signnow improve workflow for veterinary practices?
By utilizing veterinary fda1932a signnow, veterinary practices can signNowly enhance their workflow efficiency. It automates the document signing process, reduces paperwork, and minimizes delays, allowing staff to focus more on patient care rather than administrative tasks.
-
What features does veterinary fda1932a signnow include?
Veterinary fda1932a signnow offers features such as customizable document templates, multi-party signing, and secure cloud storage. These features make it easier for veterinary professionals to manage documents and ensure that all signatures are captured accurately and timely.
-
Is veterinary fda1932a signnow affordable for small veterinary clinics?
Yes, veterinary fda1932a signnow is designed to be cost-effective, making it accessible for small veterinary clinics. It offers flexible pricing plans that cater to various budgets while providing all the essential functionalities needed for effective document management.
-
Can veterinary fda1932a signnow integrate with other veterinary software?
Absolutely! Veterinary fda1932a signnow can seamlessly integrate with various veterinary software systems, enhancing overall operational efficiency. This integration enables clinics to manage their documents and client interactions without needing to switch between multiple platforms.
-
What are the benefits of using veterinary fda1932a signnow for client communication?
Veterinary fda1932a signnow enhances client communication by providing a fast and efficient way to send and receive signed documents. Clients appreciate the convenience of eSigning from any device, resulting in faster turnaround times and increased satisfaction.
-
How secure is veterinary fda1932a signnow for sensitive information?
Security is a top priority for veterinary fda1932a signnow. It employs advanced encryption and security protocols to protect sensitive information, ensuring that all documents are safe from unauthorized access and fraud while meeting industry standards.
Get more for FORM FDA 1932a Veterinary Adverse Drug Reaction, Lack Of Effectiveness, Or Product Defect Report
- Excavation contractor package massachusetts form
- Renovation contractor package massachusetts form
- Concrete mason contractor package massachusetts form
- Demolition contractor package massachusetts form
- Security contractor package massachusetts form
- Insulation contractor package massachusetts form
- Paving contractor package massachusetts form
- Site work contractor package massachusetts form
Find out other FORM FDA 1932a Veterinary Adverse Drug Reaction, Lack Of Effectiveness, Or Product Defect Report
- How To Electronic signature New Jersey Education Permission Slip
- Can I Electronic signature New York Education Medical History
- Electronic signature Oklahoma Finance & Tax Accounting Quitclaim Deed Later
- How To Electronic signature Oklahoma Finance & Tax Accounting Operating Agreement
- Electronic signature Arizona Healthcare / Medical NDA Mobile
- How To Electronic signature Arizona Healthcare / Medical Warranty Deed
- Electronic signature Oregon Finance & Tax Accounting Lease Agreement Online
- Electronic signature Delaware Healthcare / Medical Limited Power Of Attorney Free
- Electronic signature Finance & Tax Accounting Word South Carolina Later
- How Do I Electronic signature Illinois Healthcare / Medical Purchase Order Template
- Electronic signature Louisiana Healthcare / Medical Quitclaim Deed Online
- Electronic signature Louisiana Healthcare / Medical Quitclaim Deed Computer
- How Do I Electronic signature Louisiana Healthcare / Medical Limited Power Of Attorney
- Electronic signature Maine Healthcare / Medical Letter Of Intent Fast
- How To Electronic signature Mississippi Healthcare / Medical Month To Month Lease
- Electronic signature Nebraska Healthcare / Medical RFP Secure
- Electronic signature Nevada Healthcare / Medical Emergency Contact Form Later
- Electronic signature New Hampshire Healthcare / Medical Credit Memo Easy
- Electronic signature New Hampshire Healthcare / Medical Lease Agreement Form Free
- Electronic signature North Dakota Healthcare / Medical Notice To Quit Secure